Poor Prognosis
Case Objectives
- Understand the current limitations of physicians' ability to provide prognoses.
- List the variables that can be used to guide treatment decisions and prognostication in elderly patients.
- Appreciate the cognitive steps to determine prognosis in elderly patients.
Case & Commentary: Part 1
A 91-year-old woman presented with 2 days of nausea and vomiting. Physical examination revealed a palpable mass in the right groin without bowel sounds. A CT scan of the abdomen showed an incarcerated hernia complicated by small bowel obstruction. The patient was taken to the operating room for resection under general anesthesia. After extubation, she developed stridor, requiring re-intubation. Otorhinolaryngology (ENT) evaluation revealed no evidence of laryngeal edema; however, there was evidence of significant extrinsic compression of the trachea. A CT scan revealed a thyroid mass. A fine needle aspiration (FNA) biopsy was performed but was inconclusive. A repeat FNA was performed.
The attending physician held a family meeting to discuss the patient's prognosis and direction of care. He told the family the prognosis was likely very poor, as he suspected malignancy. Given this news, the family decided not to pursue surgical intervention (tracheostomy).
Physicians are frequently called upon to make predictions about expected patient survival and to disclose those predictions to patients. Research has also shown that both types of prognostic tasks are extremely difficult for physicians.
Results of a survey of a random sample of 1,311 US internists suggest that the average internist addresses the question "How long do I have to live?" ten times per year, withdraws life support five times per year, and refers patients to hospice-based palliative care five times per year.(1) Among these physicians, 60% reported that they found prognostication emotionally "stressful," and their stress with prognostication was highly associated with self-perceived prognostic inaccuracy.
Data on physicians' prognostic accuracy primarily comes from studies of physicians caring for patients already enrolled in palliative care. These studies reveal that, on average, physicians make inaccurate prognostic estimates; the direction of their error, overwhelmingly, is optimistic, with physicians overestimating survival by a factor of three.(2-8) In one study, 343 physicians provided survival estimates for 468 terminally ill cancer and non-cancer patients at the time of patient referral to hospice-based palliative care. These estimates were then compared with patients' actual survival. Physicians were accurate in their prognoses approximately 20% of the time, overestimated survival more than three times as often (63%), and underestimated survival in only a minority of instances (17%).(8)
Research has queried whether such systematic prognostic overestimation by physicians may in part explain the unexpectedly "short" survivals observed in patients referred for hospice-based palliative care. Results of the above-noted survey suggest that physicians believe an optimal length of stay in hospice is 3 months (9), yet the observed median length of stay is only 3 weeks.(8) Perhaps some of this observed inconsistency results from physicians' optimistic bias in prognostication.
This particular patient differed from those enrolled in the studies mentioned above in that she did not yet have an established "terminal illness." Since the science of prognosis is anchored in disease diagnosis and extent, this patient's diagnostic ambiguity contributed to making prognostication quite challenging. On one extreme, if the patient's neck mass was a result of anaplastic thyroid cancer (ie, a rare and rapidly fatal form of thyroid cancer), her estimated median survival would be approximately 4 months (10), and the immediate institution of supportive (and non-curative) care would be an appropriate clinical approach to managing the airway compromise. On the other hand, if her neck mass was the result of a benign goiter, her estimated median survival would probably be quite similar to her baseline age-related expected survival of approximately 4 years (11), and the institution of supportive care would not be a conventional approach to management of the airway compromise. Depending on the characteristics of the goiter (eg, diffuse, multinodular) and the approach of the endocrinologist (eg, trial of T4-suppression therapy, reductive surgery, and/or radioactive iodine), other approaches would be more conventional.
Given the very wide prognostic range associated with this patient's neck mass—4 months vs. 4 years—and the associated wide range of clinical approaches, for this patient, a tissue diagnosis would help to narrow this prognostic range and thus better define the immediate clinical approach. Although a clinician might be tempted to assume that a large mass is cancerous, studies of consecutive thyroid aspirations in community hospitals suggest that cancer explains only 5% to 6.5% (12,13) of nodules.
Case & Commentary: Part 2
After further discussion, the family decided to withdraw care, because the patient had stated previously that she did not want to be intubated for a long period. Shortly after extubation, the patient died. A few days after the patient's death, the results of the second FNA were obtained. The biopsy revealed a benign nodular goiter.
The patient, family, and physician in this vignette experienced the uncommon situation of a pessimistic prognostic error. The events described are surprising and raise an important question: why was the FNA done if its results were not going to influence care?
A natural concern in this case is whether the patient's advanced age somehow influenced the decision to pursue a less complete diagnostic approach. It is certainly hard to imagine that a 37-year-old woman would have been managed this way. However, it is possible that there were other life-limiting comorbidities (eg, a previously diagnosed advanced cancer, severe dementia, class IV congestive heart failure) and/or poor functional status that influenced her underlying or baseline prognosis and thus might explain the clinical approach.
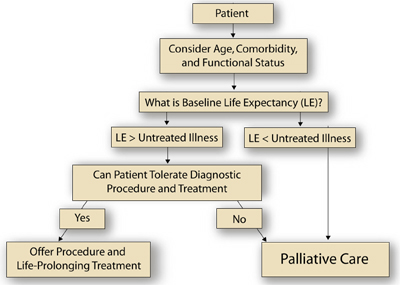
A general clinical approach to this patient can be borrowed from the field of oncology, which is currently struggling to develop systematic approaches or algorithms that acknowledge and integrate important prognostic variables (both cancer-related and non-cancer-related) to guide cancer treatment decisions in elderly patients.(14) For example, a comprehensive geriatric assessment (CGA) can yield information about functional status and comorbidities, which, along with sex and chronological age, have prognostic relevance and can be integrated to generate an estimate of baseline life expectancy.(14) The physician compares the expected survival from the untreated illness or illnesses being considered (eg, anaplastic thyroid cancer vs. benign goiter) to this estimate of baseline life expectancy. If the baseline life expectancy is greater than that of the untreated condition, the physician then needs to decide if the diagnostic procedure and/or the disease-specific treatment would result in excess morbidity and mortaility (ie, decide if the interventions are "tolerable"). If diagnostic procedure tolerance and/or treatment tolerance is deemed adequate, then the patient may benefit from further work up and, ultimately, therapy directed at the illness or the illness under consideration.
In this case, no comorbidity or functional status information is provided, but we do know that the patient was a 91-year-old woman. Life-tables indicate that 50% of 90-year-old American women will live at least an additional 3.8 years, with 25% living less than 1.8 years and 25% living at least 6.8 years. Since the expected survival from the most aggressive thyroid cancer (ie, anaplastic histology) is only 4 months and the expected survival from the most benign explanation for the neck mass (ie, benign goiter) will be unlikely to impact life-expectancy meaningfully, most algorithms would recommend biopsy. If on the other hand, the patient had a severely life-limiting illness already (eg, stage IV lung cancer), the results of the biopsy would not impact decision-making and thus would not be needed. In this latter case, supportive care for the lung cancer would be an appropriate approach. The Figure outlines an approach to such clinical decision-making for elderly patients with cancer.
When making prognostic estimates in elderly patients, it is important to consider the following issues:
- What is the diagnosis and extent of the new disease?
- What is the patient's baseline life expectancy related to age, comorbidity, and functional status?
- Is the expected survival from the new disease shorter than the baseline life expectancy?
- Will treatment improve expected survival from the new disease?
- Will treatment for the new disease be tolerated?
Prognostication is a difficult task. Most physicians are unable to accurately predict survival and are uncomfortable with the process. An evidence-based approach should be employed whenever possible, taking care to remove the effect of biases from the algorithm, but further research is needed to develop clinically useful predictive algorithms.(16-20)
Elizabeth B. Lamont, MD, MS Assistant Professor of Medicine Harvard Medical School Massachusetts General Hospital Cancer Center and Institute of Technology Assessment
Faculty Disclosure: Dr. Lamont has declared that neither she, nor any immediate member of her family, has a financial arrangement or other relationship with the manufacturers of any commercial products discussed in this continuing medical education activity. In addition, her commentary does not include information regarding investigational or off-label use of pharmaceutical products or medical devices.
References
1. Christakis NA, Iwashyna TJ. Attitude and self-reported practice regarding prognostication in a national sample of internists. Arch Intern Med. 1998;158:2389-95.[ go to PubMed ]
2. Christakis NA, Lamont EB. Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469-72.[ go to PubMed ]
3. Parkes CM. Accuracy of predictions of survival in later stages of cancer. BMJ. 1972;2:29-31.[ go to PubMed ]
4. Evans C, McCarthy M. Prognostic uncertainty in terminal care: can the Karnofsky index help? Lancet. 1985;1:1204-6.[ go to PubMed ]
5. Heyse-Moore LH, Johnson-Bell VE. Can doctors accurately predict the life expectancy of patients with terminal cancer? Palliat Med. 1987;1:165-6.
6. Forster LE, Lynn J. Predicting life span for applicants to inpatient hospice. Arch Intern Med. 1988;148:2540-3.[ go to pubmed ]
7. Maltoni M, Nanni O, Derni S, et al. Clinical prediction of survival is more accurate than the Karnofsky performance status in estimating life span of terminally ill cancer patients. Eur J Cancer. 1994;30A:764-6.[ go to PubMed ]
8. Christakis NA, Lamont EB. Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469-72.[ go to PubMed ]
9. Iwashyna TJ, Christakis NA. Attitude and self-reported practice regarding hospice referral in a national sample of internists. J Palliat Med. 1998;1:241-8.
10. Seesions RB, Burman KD. Cancer of the thyroid gland. In: Harrison LB, Sessions RB, Hong KW, eds. Head and neck cancer. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004.
11. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285:2750-6.[ go to PubMed ]
12. Belfiore A, Giuffrida D, La Rosa GL, et al. High frequency of cancer in cold thyroid nodules occurring at young age. Acta Endocrinol (Copenh). 1989;121:197-202.[ go to PubMed ]
13. Belfiore A, Giuffrida D, La Rosa GL, et al. High frequency of cancer in cold thyroid nodules occurring at young age. Acta Endocrinol (Copenh). 1989;121:197-202.[ go to PubMed ]
14. Repetto L, Granetto C, Venturino A, Rosso R, Gianni W, Santi L. Prognostic evaluation of the older cancer patient. In: Balducci L, Lyman GH, and Ershler WB, eds. Comprehensive geriatric oncology. 1st ed. Amsterdam, The Netherlands: The Netherlands Harwood Academic Publishers; 1998:287-300.
15. Balducci L. Do we need geriatric oncology? Cancer Control. 1994;1:91-93.[ go to PubMed ]
16. Lamont EB, Christakis NA. Survival estimates in advanced cancer. In: Rose BD, ed. UpToDate (versions 10.2-10.3). UpToDate. Wellesley, MA; 2002. Available at [subscription required]: [ go to related site ]. Accessed August 17, 2004.
17. Lamont EB, Christakis NA. Prognostication in advanced disease. In: Berger A, Portenoy RK, Weissman DE, eds. Principles and practice of palliative care and supportive oncology. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2002.607-14.
18. Fox E, Landrum-McNiff K, Zhong Z, Dawson NV, Wu AW, Lynn J. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease. SUPPORT Investigators. Study to understand prognoses and preferences for outcomes and risks of treatments. JAMA. 1999;282:1638-45.[ go to PubMed ]
19. Schonwetter RS, Han B, Small BJ, Martin B, Tope K, Haley WE. Predictors of six-month survival among patients with dementia: an evaluation of hospice Medicare guidelines. Am J Hosp Palliat Care. 2003;20:105-13.[ go to PubMed ]
20. Arias E. United States life tables, 2000. [National Center for Health Statistics Web site]. 2002;51:29-33. Available at: [ go to related site ]. Accessed August 17, 2004.
Figure
Figure. An Algorithm for the Treatment of Older Cancer Patients