Dissecting the Presentation
Case Objectives
- Define aortic dissection.
- Describe the epidemiology of acute aortic dissection.
- State the common and uncommon presentation of acute aortic dissection.
- Appreciate that a normal chest radiograph should not be used to rule in or out acute aortic dissection.
- List three factors leading to a missed diagnosis of aortic dissection.
- List some of the key pitfalls in the management of acute aortic dissection.
The Case
A 78-year-old woman with a past medical history of hypertension was in good health until she experienced acute onset of confusion, which resolved after a few minutes. Then she had two episodes of black and "tarry" foul-smelling diarrhea (i.e., melena, usually indicative of gastrointestinal bleeding). She was concerned about the symptoms so presented to the hospital. On presentation, she had no abdominal pain, chest pain, shortness of breath, or focal weakness in her arms or legs.
Her physical examination was notable for tachycardia. Her mental status examination was normal. Laboratory tests showed mild anemia and new acute renal insufficiency. Chest radiograph revealed some right hilar fullness but was otherwise negative, and electrocardiogram showed sinus tachycardia.
The patient was diagnosed with a transient ischemic attack and possible gastrointestinal bleeding, and she was admitted to a telemetry unit for monitoring and ongoing testing. She generally did well with no further confusion and resolving diarrhea. She did have a persistent sinus tachycardia.
On the morning of hospital day 2, she was found unconscious by the nursing staff and found to be in cardiac arrest; her cardiac rhythm was pulseless electrical activity. Despite maximal resuscitation efforts, the patient died.
Autopsy revealed the cause of death to be an acute aortic dissection (tear in the aorta) extending from the ascending aorta to the renal arteries, along with an acute hemothorax (blood in the chest cavity). The dissection was probably present on admission and the tear in the aorta had impaired blood flow leading to all of her symptoms, including the transient ischemic attack, the gastrointestinal bleeding, and the renal failure. The dissection likely worsened while the patient was hospitalized, and its rupture into her chest cavity was the terminal event.
The Commentary
In medical practice, one of the challenges is to determine whether the patient with multiple symptoms has a single unifying diagnosis. In this case, transient ischemic attack, gastrointestinal bleeding, and acute renal failure may seem unrelated, but a dissection of the aorta could, and did, explain the entire presentation through involvement of different major vessels (i.e., cerebral vessels, mesenteric artery, and renal artery, respectively).
What Is an Aortic Dissection?
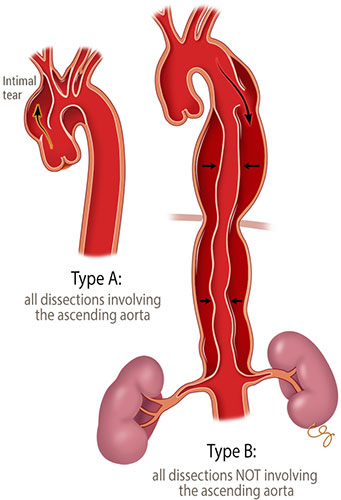
An aortic dissection is a tear in the wall of the aorta; more specifically, it is a tear of the inner and middle layers of the aortic wall with propagation of a false lumen within the middle layer.(1) Aortic dissection is part of a spectrum often referred to as acute aortic syndrome. This syndrome encompasses not only aortic dissection but also its less common variants, including aortic intramural hematoma and penetrating atherosclerotic ulcer.(2) Aortic dissection is classified using two anatomic systems. The more common (Stanford) classifies dissections that involve the ascending aorta as type A, regardless of the site of intimal tear, and all other dissections as type B (Figures).
Epidemiology
Acute aortic dissection (AAD) is rare. The true incidence is difficult to define because dissections can be instantly fatal in the prehospital setting and may be missed on initial presentation.(3) Death is often attributed to other causes, and autopsies may not be performed. We do know that there is a higher incidence of AAD in men (65%) and with increasing age.(4)
Clinical Presentation
Although rare, AAD can be associated with potentially catastrophic outcomes and a very high mortality if undiagnosed and untreated. The mortality for an untreated type A dissection is thought to be approximately 1%–2% per hour for the first 48 hours, 50% by day 3, and 80% by 2 weeks.(2,5) Type B dissections are not as serious but still have a mortality of approximately 10% at 30 days for lower-risk patients and up to 70% in high-risk groups.(2,4) As with the patient in this case, patients with AAD do not necessarily present with classic findings. The Table outlines both common and less common presentations of AAD. Up to 6.4%–12% of AAD in the thorax may be painless and present only with complications involving other body systems (e.g., abdominal pain from mesenteric ischemia).(4,6)
The classic physical examination findings for AAD (e.g., diastolic murmur, blood pressure differential between arms, focal neurologic deficit) are seen in fewer than half of all patients with AAD.(4) For example, data from the IRAD database of 2538 patients found a pulse deficit in one arm or blood pressure differential between arms in only 20% of patients.(7) Although these findings cannot be used to help rule out the presence of a dissection, their presence does increase the likelihood of the disease.(8)
In this case, the patient had transient neurologic symptoms, which can be seen in 50% of patients. While the absence of chest pain in this patient may seem unusual, it is not—fully one-third of patients with a dissection do not present with chest pain.(3)
Diagnostic Studies
The diagnosis of AAD is confirmed by advanced imaging such as ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI). Conventional chest radiographs show widening of the aorta in 63% of type A dissections, while 11% show no abnormality. The comparable values in type B dissections were 56% and 16%.(4,9) Due to the limited sensitivity of chest radiographs, especially in type B dissections, a normal chest radiograph should not be used to rule in or out AAD. In this patient, a right hilar fullness was noted. Without access to the actual chest radiograph, it is difficult to comment on how or whether this finding should have influenced the clinicians in this case.
Serum markers are emerging as a screening option. D-dimer has been examined as a screening test to rule out the presence of AAD. The evidence supporting the use of D-dimer has been mixed.(10) A meta-analysis involving seven studies showed that a D-dimer level below 0.5 μg/mL was highly sensitive (97%), and thus useful in ruling out aortic dissection.(11) Yet, one study found that 11 (18%) of 61 patients with confirmed aortic dissection had D-dimer levels below 0.4 μg/mL.(12) Currently, the 2010 American College of Cardiology Foundation and American Heart Association guidelines do not recommend using D-dimer for the evaluation of possible aortic dissection.(3) Future studies may better determine the utility of D-dimer testing.
Factors Leading to Missed Diagnosis of Aortic Dissection
The diagnosis of aortic dissection in the emergency department (ED) is missed 16% to 38% of the time.(13,14) One study identified three key factors that appeared to predispose to missing the diagnosis of AAD.(13) The foremost factor is the perceived mildness of disease at presentation. AAD was missed in 36% patients who walked into the ED compared to 13% who arrived by ambulance. A second factor leading to misdiagnosis was clinical symptoms and laboratory findings that suggested another disease. In the missed cases, acute coronary syndrome (ACS) was the most common alternative diagnosis postulated (note, a full discussion of the challenges in accurately diagnosing chest pain can be found in a previous AHRQ WebM&M commentary). A third factor was the absence of "typical" radiographic findings, such as a widened mediastinum or abnormal aortic contour, or the absence of common laboratory findings of AAD, occurring in up to 20% of patients with AAD.(4) In a different study that examined misdiagnosis of AAD in the ED, Chua and colleagues (14) found that neither age, male sex, nor a history of hypertension were significant risk factors for missed diagnosis of AAD. Instead missed diagnoses were significantly higher in the absence of pulse deficit (OR [odds ratio] 35.76, 95% CI [confidence interval] 3.70–345.34) and the absence of widened mediastinum on chest radiography (OR 33.16, 95% CI 5.74–191.49).(10) The common misdiagnoses in AAD include ACS (19%), musculoskeletal pain (20%), pneumonia or pulmonary embolism (20%), pericarditis (12%), and GI pain (9%).(15)
Risk Management Pitfalls for Acute Aortic Syndromes
Not only is the diagnosis of AAD often missed, the disease is also frequently mismanaged. A recent review article outlined pitfalls in the management of AAD.(16) According to this article, clinicians should not exclude the possibility of AAD based solely on the absence of tearing chest pain, pulse or blood pressure differential, or a widened mediastinum on chest radiograph. Importantly, as thrombolytic therapy (given for presumed stroke) can be fatal in AAD, providers should consider the possibility of AAD in patients presenting with acute neurologic deficits. Moreover, patients with AAD may present with syncope alone without chest pain; this is particularly common in older patients. It should also be kept in mind that seemingly unrelated complaints could be due to the ischemic manifestations of AAD. Emergency medicine providers should recognize that patients with chest pain and a slightly elevated troponin may not have an acute coronary syndrome but may have an AAD. Moreover, it is important to recognize that patients with AAD may present with inferior wall myocardial infarction on an electrocardiogram in 1%–7% of AAD due to involvement of the right coronary artery affecting the coronary ostia. The review (16) also stresses the need for surgical evaluation in all patients with AAD, even those with type B AAD, which are usually managed medically. All patients with AAD are at risk for rupture or organ dysfunction, both indications for surgery.
Although several studies have attempted to risk stratify patients with potential aortic dissection (3,17), no reliable prediction rules are currently available.
Better education of emergency practitioners with emphasis on the pitfalls in chest pain evaluation and AAD might decrease errors in the diagnosis of AAD. Equipping emergency physicians with the ability to do bedside echocardiography to evaluate the aortic root diameter may help to decrease Stanford type A AADs. Empowering emergency physicians to be able to easily order CT aortogram when there is a suspicion on AAD could allow for earlier identification of Stanford type A and type B AADs. In addition, emergency physicians should be made aware that CT angiography for pulmonary embolism is a different protocol from CT aortogram for AAD.
In this patient, since the acute onset of confusion was transient, TIA [transient ischemic attack] was a reasonable initial diagnosis. Concern for an acute gastrointestinal (GI) bleed was warranted in view of the melena. However, in view of the absence of shock or signs of hypoperfusion on presentation, it was likely not appropriate to attribute patient's new acute renal insufficiency to the GI bleeding. Other causes should have been sought. Since the patient did not have a known history of TIA nor was she on aspirin to account for her GI bleeding, one possible etiology to tie in all these presentations would be aortic dissection. However, to be fair, things are always clearer from a retrospectoscope. Aortic dissection of the proximal aorta involving the cerebral vessels could have caused the symptoms of TIA. Extension of the aortic dissection downwards to involve the mesenteric artery leading to mesenteric infarction as well as the formation of aortoenteric fistula or rupture into the small bowel could have caused GI bleeding. Involvement of the renal artery could have lead to acute renal insufficiency. Moreover, older patients are less likely to present with typical signs and symptoms of AAD and hence the possibility of AAD should have been entertained in this elderly patient with seemingly unrelated presenting complaints.
Take-Home Points
- Acute aortic dissection is a challenging diagnosis that may not present with the classic sudden onset of tearing or sharp chest pain or pulse deficits but with painless manifestations involving other body systems such as neurological, gastrointestinal, and renal.
- Familiarize yourself with not only the common but the uncommon presentations of acute aortic dissection and keep an open mind when patients present with multiple seemingly unrelated complaints.
- Absence of mediastinal widening on chest radiograph does not rule out aortic dissection.
- Misdiagnosis can occur when patients present with mild illness, when symptoms are suggestive of another disease (e.g., acute coronary syndrome), and when radiographic findings are not typical.
- Providers should be aware of specific pitfalls in the management of acute aortic dissection.
Shirley Beng Suat Ooi, MBBS (S'pore)
Senior Consultant and Associate Professor
Emergency Medicine Department, National University Hospital
Designated Institutional Official
National University Health System Residency Program
Singapore
Faculty Disclosure: Shirley Beng Suat Ooi has declared that neither she, nor any immediate member of her family, have a financial arrangement or other relationship with the manufacturers of any commercial products discussed in this continuing medical education activity. In addition, the commentary does not include information regarding investigational or off-label use of pharmaceutical products or medical devices.
References
1. Thrumurthy SG, Karthikesalingam A, Patterson BO, Holt PJ, Thompson MM. The diagnosis and management of aortic dissection. BMJ. 2011;344:d8290. [go to PubMed]
2. Criado FJ. Aortic dissection: a 250-year perspective. Tex Heart Inst J. 2011;38:694-700. [go to PubMed]
3. Hiratazka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation. 2010;121:e266-e369. [go to PubMed]
4. Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897-903. [go to PubMed]
5. Coady MA, Rizzo JA, Goldstein LJ, Elefteriades JA. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin. 1999;17:615-635. [go to PubMed]
6. Chen K, Varon J, Wenker OC, et al. Acute thoracic aortic dissection: the basics. J Emerg Med. 1997;15:859-867. [go to PubMed]
7. Rogers AM, Hermann LK, Booher AM, et al. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: results from the international registry of acute aortic dissection. Circulation. 2011;123:2213-2218. [go to PubMed]
8. Klompas M. Does this patient have an acute thoracic aortic dissection? JAMA. 2002;287:2262-2272. [go to PubMed]
9. von Kodolitsch Y, Nienaber CA, Dieckmann C, et al. Chest radiography for the diagnosis of acute aortic syndrome. Am J Med. 2004;116:73-77. [go to PubMed]
10. Suzuki T, Distante A, Zizza A, et al. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation. 2009;119:2702-2707. [go to PubMed]
11. Shimony A, Filion KB, Mottillo S, Dourian T, Eisenberg MJ. Meta-analysis of usefulness of D-dimer to diagnose acute aortic dissection. Am J Cardiol. 2011;107:1227-1234. [go to PubMed]
12. Paparella D, Malvindi PG, Scrascia G, et al. D-dimers are not always elevated in patients with acute aortic dissection. J Cardiovasc Med (Hagerstown). 2009;10:212-214. [go to PubMed]
13. Kurabayashi M, Miwa N, Ueshima D, et al. Factors leading to failure to diagnose acute aortic dissection in the emergency room. J Cardiol. 2011;58:287-293. [go to PubMed]
14. Chua M, Ibrahim I, Neo X, Sorokin V, Shen L, Ooi SB. Acute aortic dissection in the ED: risk factors and predictors for missed diagnosis. Am J Emerg Med. 2012;30:1622-1626. [go to PubMed]
15. Aortic dissections: "tearing" apart the data. CMPA Bulletin, R10812E; June 2008.
16. Lo BM. An evidence-based approach to acute aortic syndromes. Emerg Med Practice. 2013;15:1-23. [go to PubMed]
17. von Kodolitsch Y, Schwartz AG, Nienaber CA. Clinical prediction of acute aortic dissection. Arch Intern Med. 2000;160:2977-2982. [go to PubMed]
18. Ooi S, Chan G. Aortic emergencies. In: Ooi S, Manning P (eds). Guide to the Essentials in Emergency Medicine, 2nd ed. Singapore: McGraw-Hill; 2015:221-228. ISBN: 9780071087889.
Table
Table. Presentations of Acute Aortic Dissection.(18)
| Common Presentations |
|
|
|
|
|
|
|
| Less Common Presentations |
|
|
|
|
|
|
Figures
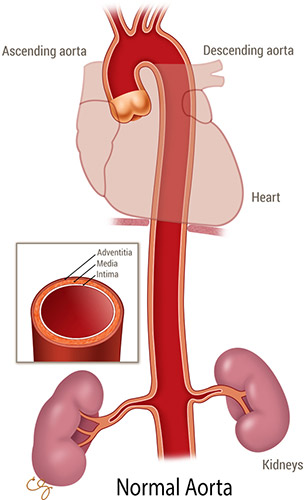
Figure 1. Normal Aorta. (Illustration © 2015 Chris Gralapp.)
Figure 2. Aortic Dissections. (Illustration © 2015 Chris Gralapp.)