Hindsight is 20/20: Thrombolytics for Alcohol Intoxication
Hindsight is 20/20: Thrombolytics for Alcohol Intoxication. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2024.
Hindsight is 20/20: Thrombolytics for Alcohol Intoxication. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2024.
Debra Bakerjian, PhD, APRN, RN; David K. Barnes, MD; Noelle Boctor, MD; Patrick Romano, MD, MPH; Eric Signoff, MD for this Spotlight Case and Commentary have disclosed no relevant financial relationships with ineligible companies related to this CME activity.
Learning Objectives
At the conclusion of this educational activity, participants should be able to:
- Appreciate stroke misdiagnosis is common and leads to serious patient harm
- Define “stroke chameleon” and “stroke mimic”
- Describe the biases that lead to stroke misdiagnosis
- Utilize bedside scoring tools (e.g., FABS) to identify “stroke mimics”
- Prioritize high-yield point-of-care labs to aid in “stroke mimic” diagnosis
The Case
A 61-year-old patient presented to the emergency department (ED) by taxicab complaining of weakness starting 4 hours before arrival. He was alert to person, time, and place, but had notable shuffling gait, slurred speech, delayed response to verbal questions, and inability to concentrate or make eye contact. His blood pressure was elevated at 156/96, but other vital signs were normal, with heart rate 71, respiratory rate 16, and temperature 37.8˚C. On physical examination, there was no facial droop, but facial symmetry was difficult to assess as he would not follow the instructions. A stroke alert was activated. The patient’s glucose was 92 mg/dL (normal). Partial gaze palsy was noted but there was no visual loss. All four limbs drifted but did not hit the bed and there was no limb ataxia. Sensory examination was normal. Aphasia was absent; dysarthria was mild-to-moderate (i.e., speech was slurred but could be understood). No extinction or inattention was observed. Together, these findings represented a National Institutes of Health Stroke Scale (NIHSS) value of 6.
A neurosurgeon evaluated the patient via teleconsult and requested neuroimaging with perfusion. Tenecteplase (TNKase) was prepared by pharmacy. There was no intracranial hemorrhage identified on non-contrast computed tomography (CT) of the head. Since the patient’s symptoms were not improving spontaneously and the treating team was suspicious of a stroke, the neurosurgeon recommended proceeding with thrombolysis. The risks and benefits were explained to the patient, and he consented. During a time-out, the clinical team verified the patient’s identity, indications for treatment, radiologic findings, consent to treatment, absent bleeding diathesis (laboratory results), TNKase checklist, and blood pressure. TNKase was administered and he was monitored per hospital protocol.
Thirty minutes after TNKase administration, laboratory tests showed that the patient’s alcohol level was 433 mg/dL, a potentially fatal level. The patient was admitted to the intensive care unit (ICU) for close monitoring. His NIHSS score increased to 7 with somnolence arousable to stimulation. A repeat CT scan was performed and revealed a new subdural hemorrhage. The neurosurgeon was updated, conservative treatment was recommended, and the patient recovered slowly.
The Commentary
By Eric Signoff, MD, Noelle Boctor, MD, and David K. Barnes, MD, FACEP
Stroke is one of five conditions linked to the largest numbers of misdiagnosis-related serious harms.1 In U.S. emergency departments, 9% of stroke patients are misdiagnosed—either under- or over-diagnosed—resulting in serious morbidity and mortality.2 Ischemic stroke remains a difficult clinical diagnosis given the many non-stroke processes that mimic stroke. Conversely, many strokes are subtle or present atypically, eluding timely diagnosis and leading to delayed care or missed treatment opportunities. This diagnostic uncertainty is compounded both by the limitations inherent to routine neuroimaging, which is unable to rule out acute ischemic stroke (AIS), and by the reduced efficacy of thrombolysis if its administration is delayed.3 Evidence supports the earliest possible administration of thrombolytic therapy for AIS if there are no contraindications. In this case, failure to diagnosis acute alcohol intoxication (AAI) mimicking stroke led to overdiagnosis and inappropriate administration of thrombolytic therapy, resulting in patient harm. Whether the administration of thrombolytic therapy to a “stroke mimic” equates to a medical error is an appropriate subject of debate.
“Stroke Chameleons” and “Stroke Mimics”
The term “stroke chameleon” refers to cases of stroke that present atypically and result in missed (i.e., underdiagnosis or false negative) or delayed diagnosis. Conversely, the term “stroke mimic” refers to cases presenting with typical stroke features that are determined, after further evaluation, to be attributable to other diagnoses (i.e., overdiagnosis or false positive).2 Examples of “stroke chameleon” are hand-knob area infarction (which can present as monoparesis), brain infarcts with convulsions, and strokes with primarily cognitive changes, such as Gerstmann syndrome, Balint syndrome, or top of the basilar syndrome.3 Posterior circulation stroke may present with dizziness and may be misidentified as a peripheral vestibulopathy.4 Examples of “stroke mimics” include migraine, functional neurologic disorder (FND) or conversion disorder, seizure, and alcohol intoxication, such as the case discussed in this commentary. Factors associated with stroke misdiagnosis include young age, female sex, minority status, and presentations with non-specific, transient, or posterior circulation symptoms.2
The historical emphasis on decreasing the underdiagnosis of stroke arose from the need to administer thrombolysis to eligible patients within a narrow time window to maximize benefit and minimize the risk of hemorrhagic complications. Overdiagnosis of stroke has received less attention, perhaps because systemic thrombolysis is associated with a much lower risk of intracranial hemorrhage when given to patients with “stroke mimic” conditions, compared to patients with AIS.5 Thus, stroke treatment guidelines recommend thrombolysis to avoid missing the therapeutic window even when a “stroke mimic” diagnosis has not been excluded; in fact, an acceptable rate of intracranial hemorrhage is recognized by neurologists.6
The diagnostic challenges associated with acute alcohol intoxication (AAI) can be partly attributed to unique pathophysiologic changes induced by ethanol. Acutely, ethanol causes not just global encephalopathy, but also impairs fibrinolysis, causes platelet activation, and increases plasminogen activator inhibitor, each increasing the risk of ischemic stroke. While moderate ethanol consumption has been suggested to lower the risk of stroke, there is ample evidence that chronic heavier alcohol use leads to increased risk of both ischemic and hemorrhagic stroke.5 Thus, patients who drink alcohol may have both elevated and lower risk of AIS, depending on their level and duration of consumption.
Diagnostic Approach to Altered Mental Status and Diagnostic Error in Stroke
The bedrock of clinical diagnosis is the patient history. When the patient’s history is unreliable or unobtainable, such as in cases of altered mental status, collateral history is essential to inform diagnostic reasoning.7 Alternate sources of history include friends, family, bystanders, emergency medical services, and the electronic health record.
The differential diagnosis of altered mental status is broad, and a full examination of its causes is beyond the scope of this commentary. Nevertheless, AAI is a common cause of altered mental status and is a well-known “stroke mimic.” In the U.S., AAI is responsible for 1.2% of annual visits to emergency departments and is the eighth leading cause of preventable death.8 Diagnostic criteria for AAI include historical elements (e.g., recent alcohol ingestion), specific clinical changes (e.g., slurred speech), and exclusion of other medical conditions that better explain the clinical findings.9 The odor of alcohol may be a helpful finding,10 but must be interpreted cautiously due to the risk of mislabeling non-intoxicated patients. AAI may mimic a posterior circulation stroke, presenting with symptoms such as slurred speech, gait disturbances, and nystagmus, much like the symptoms seen in this case.5 Chronic alcohol use may cause subacute encephalopathy presenting with seizure-like activity and/or transient focal neurologic deficits, also mimicking stroke.3
While the clinical history and physical examination often suggest AAI, diagnosis is difficult without objective laboratory data when collateral history is unavailable. Therefore, measurement of blood or breath alcohol levels remains the most definitive means of diagnosis.8
Case Factors Impacting Accurate Diagnosis
Telemedicine is the delivery of healthcare-related clinical services via electronic and telecommunication methods, eliminating the need for an in-person encounter.11,12 By eliminating the need for travel, telemedicine is more convenient for many patients. For those in rural areas and those who face transportation barriers, telemedicine improves access to care. Similarly, rural hospitals that lack subspecialty coverage benefit from telemedicine services provided by remote clinicians.13 The use of telemedicine—telestroke in this instance—evaluation suggests that the stroke specialist was not on site to examine the patient. Given that a telestroke neurologic exam is not the same as an in-person exam, the use of this platform raises questions regarding the accuracy of remote evaluations when critical diagnoses are being considered.
By expanding access to stroke specialists at community and rural hospitals that lack in-person stroke specialist coverage, telemedicine can expand the use of thrombolysis as a stroke treatment.13 Recent evidence suggests high levels of agreement (90%) between telestroke and in-person evaluation regarding thrombolysis decisions in stroke.15 While telestroke services significantly increase the opportunity to treat stroke when experts are not physically present, the earlier evaluation afforded by telestroke increases the risk of overdiagnosis and inappropriate treatment of a “stroke mimic.”15,16
Tyranny of Metrics
Evidence supports the emergent administration of intravenous thrombolysis for acute ischemic stroke to prevent major disability.17 Indeed, one key process measure required by the Joint Commission and the American Heart Association for Comprehensive Stroke Center certification is administration of thrombolytic therapy to eligible patients within 60 minutes of ED arrival (aka, “door to needle” time).18 However, pressure to meet this time standard must be measured against the potential harm when thrombolysis is administered to a patient who is later determined to have presented with a “stroke mimic.”
Approximately 30% of patients admitted for stroke are later diagnosed with a different presenting condition. Despite the relatively low risk of intracranial hemorrhage among patients with “stroke mimic” conditions who receive thrombolysis, inappropriate thrombolytic therapy still increases the risk of hemorrhage and is associated with delayed diagnosis of the actual underlying condition.19 Therefore, a patient with a “stroke mimic” who receives thrombolysis is considered to have been the subject of a diagnostic medical error, even if no harm resulted. But what led to the diagnostic error in this case? The clinicians carefully evaluated the patient and even completed a checklist before administering TNKase.
While diagnostic uncertainty is not unique to stroke, the time-dependent nature of AIS therapy—amidst calls for ever-faster door-to-needle times—juxtaposed with the lack of a confirmatory study for AIS (i.e., ischemic stroke remains a clinical diagnosis) contributed to the error in this case. Given two diagnostic possibilities—stroke and a non-stroke etiology—conventional clinical reasoning supports treating the most time-sensitive condition if the treatment window is narrow, if withholding treatment would cause harm, if the risk of treatment is acceptably low, and if not treating the alternate condition can be delayed. Indeed, administration of thrombolytics in “stroke mimics” is supported by the American Heart Association with a Class IIa recommendation: “The risk of symptomatic intracranial hemorrhage in the stroke mimic population is quite low; thus, starting IV alteplase is probably recommended in preference over delaying treatment to pursue additional diagnostic studies.”20
Laboratory Testing
Inherent to this discussion is whether the hospital in this case had the capability to measure serum ethanol quickly. Standard laboratory testing in patients presenting with AIS includes only the point-of-care blood glucose. According to the American Heart Association and American Stroke Association guidelines, other tests such as the international normalized ratio (INR), activated partial thromboplastin time (APTT), and platelet count “may be necessary in some circumstances if there is suspicion of coagulopathy.” It is not customary to measure and/or wait for other laboratory tests; “alteplase treatment should not be delayed while waiting for hematologic or coagulation testing if there is no reason to suspect an abnormal test” (INR is an exception in patients who are anticoagulated).20
It is possible that TNKase would not have been administered to this patient had the serum ethanol been measured and reported earlier. However, the presence of high serum ethanol concentration does not exclude the possibility of a concomitant stroke—intoxicated patients can also have strokes. Conceivably, had they known the patient was intoxicated, the care team may have altered their decision making. Armed with the knowledge that thrombolysis in the setting of a “stroke mimic” is associated with a low rate of hemorrhage, a conservative clinician might still administer thrombolysis if the “stroke mimic” symptoms were convincing, and the stroke treatment window was expected to close before intoxication resolved.
Heuristics and Biases
The pressure to make rapid decisions regarding thrombolysis in a patient presenting with a possible stroke involves a dual-process cognitive model coined by psychologists Daniel Kahneman and Amos Tversky: System 1 thinking (i.e., automatic thinking) and System 2 thinking (i.e., reasoning). In emergent cases, clinicians rely on heuristics (System 1 thinking): fast, automatic, and somewhat unconscious decision making. While this system permits cognitive offloading and expedites critical decisions, it may be more compromised by biases, especially in the hands of clinicians who are relatively inexperienced or have limited access to the patient.2,21
Biases can infiltrate multiple phases of the diagnostic and therapeutic process. For example, clinician judgement can be influenced by factors that easily come to mind due to availability bias. In these cases, “cannot miss” diagnoses, such as stroke, rise to the top of differential. Clinicians in these high risk, high reward situations may also fall prey to commission bias, which is the tendency, in the midst of uncertainty, to err on the side of action regardless of the evidence. Anchoring bias occurs when information obtained early in the diagnostic process is weighted disproportionately in clinical reasoning, while later findings that contradict earlier information are ignored or downplayed.
Atypical Features of Case
The focal neurologic deficits seen in this patient due to AAI are atypical and may have contributed to the error. In cases of diagnostic uncertainty concerning stroke, the use of clinical decision tools may guide clinicians to an accurate diagnosis. Two such tools have been shown to assist in differentiating “stroke mimics” from true strokes: the FABS score and the Telestroke mimic score (TM-score).
The FABS score is a six-point scale used to identify non-stroke patients. One point is given for the each of the following:22
- Absence of facial droop (F)
- Negative history of atrial fibrillation (A)
- Age < 50 years (A)
- Systolic blood pressure < 150 mmHg at presentation (B)
- History of seizures (S)
- Isolated sensory symptoms without weakness (S)
The higher the score, the more likely a “stroke mimic.” A FABS score ≥ 3 has a 90% sensitivity and 91% specificity for identifying a “stroke mimic.”2 Without collateral information, it would be challenging to apply the FABS score to this patient. However, he probably would have scored low risk for stroke: 1 point for the absence of facial droop, and 2 additional points if he did not have a history of atrial fibrillation or seizures. The developers of the score reported five posterior circulation strokes that presented with high FABS scores, suggesting caution in its use in patients with predominantly posterior circulation signs such as gait/truncal ataxia, dysphagia, or abnormal cough.23
The Telestroke mimic score (TM-score) resembles the FABS score but was developed for telemedicine consultations, which is more appropriate for the current case. The TM-score is determined using the following criteria:24
- Age (age x 0.2 points)
- History of atrial fibrillation = +6 points
- History of hypertension = +3 points
- History of seizures = -6 points
- facial weakness = +9 points
- NIHSS > 14 = + 5 points
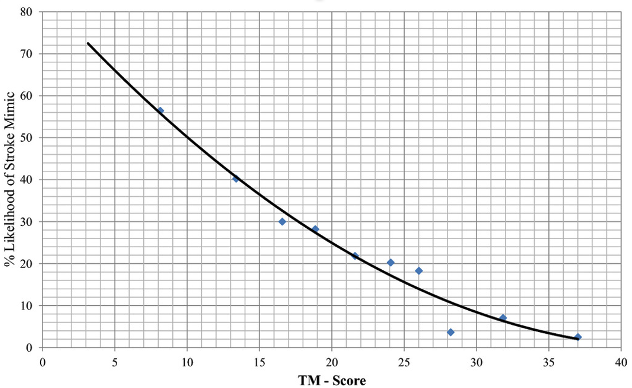
A lower TM-score is associated with a “stroke mimic”: ≤ 5 points strongly suggests a “stroke mimic,” while a score ≥ 20 strongly supports the diagnosis of stroke. Subsequent validation studies have supported these initial findings, highlighting scores <10 points as supporting a “stroke mimic” diagnosis.25 In the setting of altered mental status and lack of collateral history or medical records, much of the TM-score cannot be determined in this case, nor would it have been diagnostically helpful. Assuming he did not have a history of atrial fibrillation, hypertension, or seizures, his TM-score would be 12.2, corresponding to approximately 44% chance of a “stroke mimic” (Figure 1).
Figure 1. Relationship between TeleStroke Mimic (TM)-Score and the Likelihood of Having Stroke Mimic
Source: Image from Ali SF, Viswanathan A, Singhal AB, et al. The TeleStroke mimic (TM)-score: a prediction rule for identifying stroke mimics evaluated in a Telestroke Network. J Am Heart Assoc. 2014;3(3):e000838.
Approach to Improving Safety & Patient Safety Target
A few system changes could reduce the risk of similar missed diagnostic opportunities in the future. While the adage “time is brain” is accurate in the management of AIS, an emphasis on collateral information is important when the medical history is inadequate or unavailable. Attempts to obtain collateral sources of history should be implemented quickly using all available sources. The widespread availability of electronic health information exchanges facilitates searching a patient’s past medical history to obtain a more comprehensive picture of their clinical presentation.
Although the medical history and the physical examination are the bedrock of clinical reasoning, patients often present with ambiguous or atypical signs and symptoms, or with multiple disease processes simultaneously. In such cases of diagnostic uncertainty, clinical decision-making tools embedded into the electronic health record, such as the FABS or TM-score, can assist with the diagnostic evaluation.
Practice-changing laboratory information should be considered for high-risk clinical conditions like stroke. The use of point-of-care laboratory panels for glucose, hemoglobin, and electrolytes helps to rule out several “stroke mimics” quickly. Point of care ethanol levels could provide similar clarity. If the point-of-care ethanol level is unobtainable, one should prioritize lab tests such as the complete blood count, basic metabolic panel, blood ethanol level, and urine drug screen early after patient arrival so that results are available to guide management as soon as possible.
Emerging imaging technologies may be useful in patients presenting with stroke symptoms, especially in those with a lower likelihood of stroke. Hyperacute magnetic resonance imaging (MRI) is used to identify brain lesions consistent with stroke, before administering thrombolytics, in cases where the clinician has lower suspicion for the diagnosis. Some centers have incorporated hyperacute MRI for all acute stroke patients. However, this modality may not be available in many hospitals given its high cost. More studies evaluating the accuracy and cost-effectiveness of hyperacute MRI for detection of stroke are needed.2
Take-Home Points
- “Stroke chameleons” are strokes that present atypically leading to missed or delayed diagnosis (underdiagnosis or false negatives).
- “Stroke mimics” are non-stroke conditions that present like strokes and lead to stroke overdiagnosis (false positives).
- The workup of patients with altered mental status with suspected stroke should include direct and collateral history, physical examination, clinical decision support tools, and point of care laboratory tests (starting with blood glucose) to rule out “stroke mimics.”
- Rapid measurement of blood alcohol level may reduce the rate of stroke overdiagnosis in patients with alcohol intoxication presenting with stroke symptoms.
- Telestroke services can increase access to stroke specialist care and expand availability of thrombolytic therapy for acute ischemic stroke but may lead to overdiagnosis and overtreatment.
- Hyperacute MRI is an emerging imaging modality that may improve diagnostic uncertainty inherent to certain stroke presentations.
Eric Signoff, MD
Health Sciences Clinical Associate Professor
Department of Internal Medicine, Division of Hospital Medicine
UC Davis Health
esignoff@ucdavis.edu
Noelle Boctor, MD
Consulting Editor, AHRQ, Patient Safety Network (PSNet)
Health Sciences Clinical Assistant Professor
Department of Internal Medicine, Division of Hospital Medicine
UC Davis Health
nboctor@ucdavis.edu
David K. Barnes, MD, FACEP
Consulting Editor, AHRQ, Patient Safety Network (PSNet)
Health Sciences Clinical Professor
Director of Faculty Development
Director of ED Sustainability
Department of Emergency Medicine
Physician Advisor
UC Davis Health
dbarnes@ucdavis.edu
References
- Newman-Toker DE, Nassery N, Schaffer AC, et al. Burden of serious harms from diagnostic error in the USA. BMJ Qual Saf. 2024;33(2):109–120. [Free full text]
- Bakradze E, Liberman AL. Diagnostic error in stroke—reasons and proposed solutions. Curr Atheroscler Rep. 2018;20(2):11. [Available at]
- Hextrum S, Biller J. Clinical distinction of cerebral ischemia and triaging of patients in the emergency department: mimics, wake-ups, late strokes, and chameleons. Neuroimaging Clin North Am. 2018;28(4):537–549. [Available at]
- Newman-Toker DE, Edlow JA. TiTrATE: a novel, evidence-based approach to diagnosing acute dizziness and vertigo. Neurol Clin. 2015;33(3):577–599. [Free full text]
- Hassing LT, Verschoof MA, Koppen H. Alcohol intoxication as a stroke mimic and the incidence of acute alcohol intoxication in stroke. J Stroke Cerebrovasc Dis. 2019;28(12):104424. [Available at]
- Saver JL, Barsan WG. Swift or sure?: The acceptable rate of neurovascular mimics among IV tPA-treated patients. Neurology. 2010;74(17):1336-1337. [Available at]
- Fitzpatrick D, Doyle K, Finn G, et al. The collateral history: an overlooked core clinical skill. Eur Geriatr Med. 2020;11(6):1003–1007. [Free full text]
- Mirijello A, Sestito L, Antonelli M, et al. Identification and management of acute alcohol intoxication. Eur J Intern Med. 2023;108:1–8. [Free full text]
- American Psychiatric Association. (2022). Bipolar and related disorders. In Diagnostic and Statistical Manual of Mental Disorders (5th ed., text rev.). [Available at]
- Malhotra S, Kasturi K, Abdelhak N, et al. The accuracy of the olfactory sense in detecting alcohol intoxication in trauma patients Emerg Med J. 2013;30(11):923-925. [Available at]
- Commission FC. Telehealth, Telemedicine, and Telecare: What’s What? [Internet]. (Accessed Feb 19, 2024). [Free full text]
- Gajarawala SN, Pelkowski JN. Telehealth benefits and barriers. J Nurse Pr. 2021;17(2):218–221. [Free full text]
- Haleem A, Javaid M, Singh RP, et al. Telemedicine for healthcare: capabilities, features, barriers, and applications. Sens Int. 2021;2:100117. [Free full text]
- Lee VH, Howell R, Yadav R, et al. Thrombolysis of stroke mimics via telestroke. Stroke Vasc Neurol. 2022;7(3):267–270. [Free full text]
- Bowry R, Parker S, Rajan SS, et al. Benefits of stroke treatment using a mobile stroke unit compared with standard management: The BEST-MSU Study run-in phase. Stroke. 2015;46(12):3370-3374. [Free full text]
- Kepplinger J, Barlinn K, Deckert S, et al. Safety and efficacy of thrombolysis in telestroke: a systematic review and meta-analysis. Neurology. 2016;87(13):1344–1351. [Available at]
- Waseem H, Salih YA, Burney CP, et al. Efficacy and safety of the telestroke drip-and-stay model: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2021;30(4):105638. [Available at]
- American Heart Association. Get With the Guidelines: Stroke Program Measures & Definitions [Internet]. (accessed February 19, 2024) [Free full text (PDF)]
- Anathhanam S, Hassan A. Mimics and chameleons in stroke. Clin Med. 2017;17(2):156–160. [Free full text]
- Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e99. [Free full text]
- Kahneman D, Klein G. Conditions for intuitive expertise: a failure to disagree. Am Psychol. 2009;64(6):515-526. [Available at]
- Goyal N, Tsivgoulis G, Male S, et al. FABS: an intuitive tool for screening of stoke mimics in the emergency department. Stroke. 2016;47(9):2216-2220. [Free full text]
- Alemseged F, Rocco A, Arba F, et al. Posterior National Institutes of Health Stroke Scale improves prognostic accuracy in posterior circulation stroke. Stroke. 2022;53(4):1247-1255. [Free full text]
- Ali SF, Viswanathan A, Singhal AB, et al. The TeleStroke mimic (TM)-score: a prediction rule for identifying stroke mimics evaluated in a Telestroke Network. J Am Heart Assoc. 2014;3(3):e000838. [Free full text]
- Carlin R, Zhang N, Demaerschalk BM. Validation of the Telestroke Mimic Score in Mayo Clinic population. J Stroke Cerebrovasc Dis. 2021;30(10):106021. [Available at]