Hyperbilirubinemia Refractory to Phototherapy
Bhutani VK, Wong RJ. Hyperbilirubinemia Refractory to Phototherapy. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2017.
Bhutani VK, Wong RJ. Hyperbilirubinemia Refractory to Phototherapy. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2017.
The Case
A 1-day-old full-term infant was noted to have elevated total serum bilirubin (TSB) of 14.7 mg/dL (251 μmol/L) due to hemolytic disease of the newborn (ABO incompatibility). The patient was placed on a mattress with embedded phototherapy lights in the mother's postpartum room. Despite the treatment, the TSB level continued to climb, reaching 18 mg/dL (308 μmol/L).
The patient was transferred to the neonatal intensive care unit and staff began preparations for an exchange transfusion. In the meantime, a neonatologist asked that the irradiance level of the phototherapy lights be tested, and it was found to be well below the recommended level. The lights were replaced. The infant's TSB level began to drop and the exchange transfusion was aborted.
Further investigation revealed that there had been no regular testing of phototherapy light irradiance and that some of the unit's equipment had aging lights with insufficient irradiance levels. A protocol was put in place to ensure: (i) testing of irradiance levels prior to each use of the phototherapy mattresses or other device; (ii) routine monthly irradiance level testing of all phototherapy devices; and that a label is placed on each device with the date and time of the last test.
The Commentary
by Vinod K. Bhutani, MD, and Ronald J. Wong
This near-miss event reveals common misconceptions regarding the use of phototherapy, the most frequently prescribed medical intervention for newborn jaundice. Phototherapy is usually successfully managed by pediatric clinical teams within normal well-infant practices. However, cases such as the one described underscore the need for the implementation of guidelines to ensure safe and effective phototherapy.
Evidence has shown that screening using hour-specific bilirubin measurements (1) and consideration of clinical risk factors can identify infants who are likely to develop severe neonatal hyperbilirubinemia.(2) Screening for at-risk infants consists of determining an infant's age of jaundice onset, performing clinical risk assessment, and measuring bilirubin levels (either in total serum bilirubin [TSB] or by transcutaneous [TcB] estimation if TSB 3,4) Jaundice is present in 84% of healthy newborns.(5) The infant's predischarge bilirubin level and gestational age can be used to assess the risk of developing significant hyperbilirubinemia.(4) Predischarge TcB/TSB (adjusted for postnatal age) combined with specific clinical factors, especially earlier gestational age, bruising, positive direct antiglobulin test, Asian race, exclusive breastfeeding, blood type incompatibility, and jaundice extent, predicted subsequent phototherapy use.(5)
Phototherapy remains the best approach for the prevention and treatment of severe neonatal hyperbilirubinemia (TSB >20 mg/dL [342 μmol/L]).(1,6) However, it is crucial that phototherapy is applied correctly to combat increased bilirubin production.(1,6,7) The wavelength range of the emitted light source of a phototherapy device must overlap with the action spectrum of bilirubin to be effective.(1) Light in the wavelength range of 450–475 nm (blue) is the most effective because it overlaps the peak absorption spectrum of bilirubin. On the other hand, broadband light (such as fluorescent or halogen light) contains ultraviolet (UVA and UVB) light, which is harmful and must be avoided.(8-10) The efficacy of phototherapy is dependent on the light source (including its configuration [overhead or blanket/wraps] and irradiance), duration of exposure, and exposed body surface area of an infant. For optimal phototherapy, the light source should emit light in the blue-to-green range (~460–490 nm), produce an irradiance of at least 30 μr/cm2/nm (confirmed with an appropriate irradiance meter), and illuminate the maximal body surface area (80%) of an infant.(6) Therapeutic photon-bilirubin interaction occurs within 15 to 30 minutes of light exposure, while decreases in TSB levels occur within 4 to 6 hours of light exposure.(9,10)
Phototherapy use in the United States ranged from 4% to 14% of all healthy newborns cared for by clinicians relying on 2004 American Academy of Pediatrics (AAP) Guidelines.(1,3,11) The current AAP recommendations for the initiation of phototherapy are based on open-access TSB thresholds.(12) Studies on the reported implementation of routine bilirubin screening showed that when prophylactic use of phototherapy was performed on live births under 35 weeks of gestational age led to drastically reduced need for exchange transfusions (from 3.6 to 1.9 per 100,000 live births).(13,14) However, accurate laboratory assays need to be integrated into clinical guidelines to better translate individual risk of phototherapy to predictive risk of an appropriate measure of bilirubin toxicity.(13,14)
Significant neonatal hyperbilirubinemia can present as bilirubin-induced neurologic dysfunction (BIND).(15) This complication can be prevented by appropriate use of phototherapy. Delivery devices are designed to deliver appropriate wavelength light with adequate intensity and uniform light distribution, and also to adhere to standards of safety. Thus, the application of phototherapy should comport with universal prescription guidelines and be adjusted for infant maturity (age). Recent studies have shown that infants with thin and translucent skin may be more vulnerable to the oxidants generated by light exposure.(16,17) Conversion of bilirubin to more water-soluble byproducts by 20%–25% of TSB within 1–2 hours could have a direct neuroprotective effect.(18) Thus, the immediate and effective (crash-cart) phototherapy in the medical emergency of extreme neonatal jaundice could also reverse concurrent bilirubin neurotoxicity among newborns with mild to moderate acute bilirubin encephalopathy.(18)
With earlier and effective phototherapy, exchange transfusions are usually preventable. Yet, the following infants are most likely undergo the procedure: infants exhibiting signs of BIND, mild to moderate acute bilirubin encephalopathy, or infants with extreme hyperbilirubinemia (TSB >25 mg/dL [427 μmol/L]) that is unresponsive to effective phototherapy.(1,3) Exchange transfusions should be performed only by trained personnel in a neonatal intensive care unit with full monitoring and resuscitation capabilities. Delivery of appropriate phototherapy has decreased the need for such risk-laden exchange transfusions, although continued inquiry is needed to reach optimal risk versus benefits among certain vulnerable term infants.
Because the application of phototherapy is simple and relatively easy, we must be cautious about its misuse, overuse, or abuse in the treatment of neonatal hyperbilirubinemia. First, light should be considered a "drug" (19); thus, accurate measurements of irradiance are a must. Second, phototherapy should be adjusted for use in infants with fragile skin, low antioxidant reserves, and disordered bilirubin–albumin binding.(16,17,20) Once TSB levels are below levels associated with bilirubin neurotoxicity, phototherapy does not need to be applied continuously and may be delivered intermittently, with the off time dedicated for breastfeeding and parent–infant bonding.
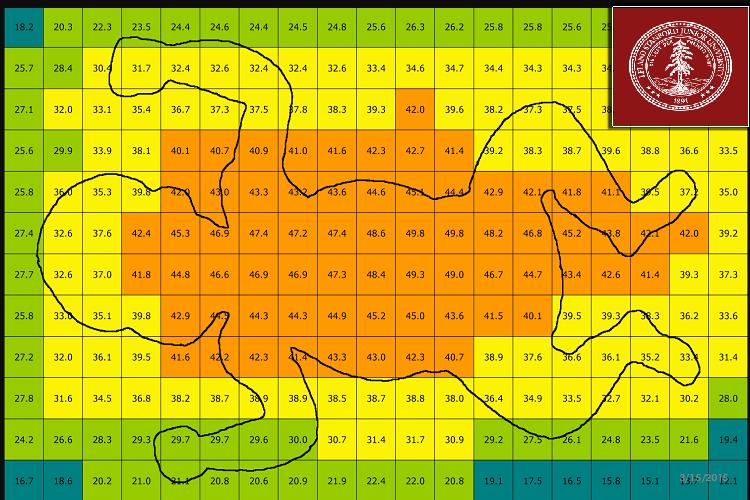
To measure irradiance, only light meters supplied by the manufacturer should be used. Visual estimations of brightness and use of ordinary photometric or colorimetric light meters are not recommended.(6) Maximal irradiance can be achieved by bringing the light source closer to the infant. However, devices that use halogen or tungsten lamps as light sources should not be moved closer because these devices can generate excessive heat and may burn the infant. Furthermore, with some devices, increasing the proximity may reduce an infant's exposed body surface area. Because the irradiance distribution to the illuminated area (footprint) is rarely uniform, measurements at the center of the footprint may greatly exceed those at the periphery and can be highly variable among phototherapy devices.(Figure) Thus, irradiance should be measured at several sites on the infant's body.
When the phototherapy lamps in this case were tested, their irradiance was well below recommended levels. The irradiance of all lamps decreases with use and age. Manufacturers' useful-lifetime estimates should not be exceeded. In current practice, phototherapy effectiveness is not based on number of devices used. A licensed physician or nurse practitioner should prescribe phototherapy by specifying a desired irradiance, light wavelength, and exposed body surface area.
In this case, multidisciplinary and institutional roles and responsibilities for effective phototherapy were not clear. Proper patient management requires an appropriate and well-trained team, including physicians, nurses, patient educators, and biomedical engineers where available. With early detection, intervention and systems approachs, the number of infants with severe hyperbilirubinemia and those who need phototherapy are likely to decrease.
Take-Home Points
- Identify neonates with increased bilirubin production for rapid progression of total serum bilirubin and need for urgent evaluation and intervention.
- The current American Academy of Pediatrics recommendations for initiation of phototherapy are based on open-access total serum bilirubin thresholds.
- Implement strategies to prevent exchange transfusion through root cause analysis using a systems approach.
- Use irradiance meters supplied by manufacturers to monitor performance of phototherapy devices.
Vinod K. Bhutani, MD Professor, Department of Pediatrics Division of Neonatal and Developmental Medicine Stanford University School of Medicine Lucile Packard Children's Hospital Stanford Children's Health Stanford, CA
Ronald J. Wong Senior Research Scientist, Department of Pediatrics Division of Neonatal and Developmental Medicine Stanford University School of Medicine Lucile Packard Children's Hospital Stanford Children's Health Stanford, CA
References
1. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316. [go to PubMed]
2. Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant > or =35 weeks' gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198. [go to PubMed]
3. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.[go to PubMed]
4. Keren R, Luan X, Friedman S, Saddlemire S, Cnaan A, Bhutani VK. A comparison of alternative risk-assessment strategies for predicting significant neonatal hyperbilirubinemia in term and near-term infants. Pediatrics. 2008;121:e170-e179. [go to PubMed]
5. Bhutani VK, Stark AR, Lazzeroni LC; Initial Clinical Testing Evaluation and Risk Assessment for Universal Screening for Hyperbilirubinemia Study Group. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J Pediatr. 2013;162:477-482. [go to PubMed]
6. Bhutani VK; Committee on the Fetus and Newborn; American Academy of Pediatrics. Phototherapy to prevent severe neonatal hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2011;128:e1046-e1052. [go to PubMed]
7. Stevenson DK, Fanaroff AA, Maisels MJ, et al. Prediction of hyperbilirubinemia in near-term and term infants. Pediatrics. 2001;108:31-39. [go to PubMed]
8. Cline BK, Vreman HJ, Faber K, et al. Phototherapy device effectiveness in Nigeria: irradiance assessment and potential for improvement. J Trop Pediatr. 2013;59:321-325. [go to PubMed]
9. Maisels MJ, McDonagh AF. Phototherapy for neonatal jaundice. N Engl J Med. 2008;358:920-928.[go to PubMed]
10. Vreman HJ, Wong RJ, Stevenson DK. Phototherapy: current methods and future directions. Semin Perinatol. 2004;28:326-333. [go to PubMed]
11. Bhutani VK, Zipursky A, Blencowe H, et al. Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res. 2013;74(suppl 1):86-100. [go to PubMed]
12. Longhurst C, Turner S, Burgos AE. Development of a web-based decision support tool to increase use of neonatal hyperbilirubinemia guidelines. Jt Comm J Qual Patient Saf. 2009;35:256-262. [go to PubMed]
13. Bhutani VK, Meng NF, Knauer Y, et al. Extreme hyperbilirubinemia and rescue exchange transfusion in California from 2007 to 2012. J Perinatol. 2016;36:853-857. [go to PubMed]
14. Kuzniewicz MW, Greene DN, Walsh EM, McCulloch CE, Newman TB. Association between laboratory calibration of a serum bilirubin assay, neonatal bilirubin levels, and phototherapy use. JAMA Pediatr. 2016;170:557-561. [go to PubMed]
15. Bhutani VK, Wong RJ. Bilirubin-induced neurologic dysfunction (BIND). Semin Fetal Neonatal Med. 2015;20:1. [go to PubMed]
16. Morris BH, Oh W, Tyson JE, et al; NICHD Neonatal Research Network. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. N Engl J Med. 2008;359:1885-1896. [go to PubMed]
17. Stevenson DK, Wong RJ, Arnold CC, Pedroza C, Tyson JE. Phototherapy and the risk of photo-oxidative injury in extremely low birth weight infants. Clin Perinatol. 2016;43:291-295. [go to PubMed]
18. Hansen TWR. The role of phototherapy in the crash-cart approach to extreme neonatal jaundice. Semin Perinatol. 2011;35:171-174. [go to PubMed]
19. Lamola AA. A pharmacologic view of phototherapy. Clin Perinatol. 2016;43:259-276. [go to PubMed]
20. Arnold C, Pedroza C, Tyson JE. Phototherapy in ELBW newborns: does it work? Is it safe? The evidence from randomized clinical trials. Semin Perinatol. 2014;38:452-464. [go to PubMed]
21. Vreman HJ, Wong RJ, Murdock JR, Stevenson DK. Standardized bench method for evaluating the efficacy of phototherapy devices. Acta Paediatr. 2008;97:308-316. [go to PubMed]