Right Regimen, Wrong Cancer: Patient Catches Medical Error
Weingart SN, Jacobson J. Right Regimen, Wrong Cancer: Patient Catches Medical Error. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2013.
Weingart SN, Jacobson J. Right Regimen, Wrong Cancer: Patient Catches Medical Error. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2013.
Case Objectives
- Appreciate that chemotherapy administration is hazardous and challenging.
- Describe the most common types of errors associated with chemotherapy administration.
- State why errors may be common when chemotherapy is administered in the inpatient setting.
- Describe the importance of understanding the process of chemotherapy administration and the importance of standardizing the process.
Case & Commentary—Part 1
A 48-year-old man with a history of metastatic penile cancer was admitted to an inpatient internal medicine service for his fourth round of chemotherapy. He had three previous uncomplicated admissions where he received a standard protocol of 3 days of paclitaxel, ifosfamide, and cisplatin. The patient received this regimen for 3 days with minimal adverse effects. On hospital day 4, based on his previous admissions for chemotherapy, the patient was expecting to go home. In the morning his bedside nurse for the day came in and stated that she would be giving him his fourth day of chemotherapy. The patient was surprised by this and, before the chemotherapy was administered, asked to speak with the oncology team who was directing his care. After speaking with the patient, the oncology fellow examined the orders in more detail and realized that the incorrect chemotherapy regimen had been ordered for the patient. Rather than the 3-day regimen for metastatic penile cancer, the order set for a higher dose 5-day regimen of paclitaxel, ifosfamide, and cisplatin for germ cell cancer had been ordered. She and the attending oncologist discussed this with the patient and he was discharged later that day with no adverse consequences.
Oncology care is dangerous business. Patients with cancer have a potentially life threatening disease and often require toxic therapies for palliation or cure. Chemotherapy, a common treatment modality, requires expertise in all stages of the medication process as well as meticulous coordination of care. Oncology team members of various specialties and professions (including physicians, nurses, pharmacists, and others) often work in different areas of a hospital or clinic, deliver asynchronous care, and follow complex treatment regimens. These regimens can involve multiple drugs administered in repetitive cycles, and are adjusted periodically to address toxicities. As the case illustrates, chemotherapy administration is among the more hazardous and challenging activities in all of medicine.
In light of the operational challenges of managing chemotherapy safely, there is surprisingly little epidemiologic evidence about the extent or nature of medical errors in chemotherapy care. In the Harvard Medical Practice Study, researchers examined medical records of 30,000 patients hospitalized in the state of New York in 1984 for medical errors and injuries. Only 3% of adverse drug events (ADEs) were attributed to "anti-tumor" medications, and these were generally deemed "unavoidable."(1) In a similar study of 15,000 patients hospitalized in Colorado and Utah in 1992, only 1.4% of ADEs were attributable to cancer chemotherapy, and none were judged to be errors.(2) However, in a seminal study of 4000 inpatients at two Boston hospitals, ADEs related to antineoplastic agents accounted for 7% of ADEs, but only 3% to 4% of medication errors.(3)
Several studies have focused specifically on chemotherapy errors in ambulatory settings, where the majority of cancer care is delivered. In a 2000 study conducted at a Boston cancer center, researchers examined more than 3200 chemotherapy orders for adult and pediatric patients. Errors were more common in drug ordering compared with administration or dispensing. The chemotherapy error rate was 3%, 2% of which had the potential to cause harm.(4) About half were intercepted by pharmacists and nurses, and no injuries occurred. Common chemotherapy-related errors included missed doses, "live" orders on days when clinicians decided to defer treatment, and failure to specify the blood counts and other laboratory test results needed to initiate therapy. In a more recent study of nearly 1400 adult and pediatric patient visits to four United States cancer centers or clinics, investigators reported chemotherapy error rates of 0.3 to 5.8 per 100 visits (depending on the site).(5) Errors most commonly occurred in administration (56%); having two concurrent sets of active orders was a particularly frequent source of error. In this scenario, one set of orders was written at the time of diagnosis and another, with adjusted doses, was written on the day of administration. A common theme in these studies is the relatively low error rate compared with non-chemotherapy medications, and the presence of errors related to redundant, confusing, or incompletely specified orders.
Two medication classes have attracted particular interest with respect to safe administration. Inadvertent intrathecal (into the cerebrospinal fluid) vincristine administration is nearly uniformly fatal as this agent is a potent neurotoxin. In a review of 41 such cases, adverse events were often related to the use of look-alike medication syringes and co-administration of intravenous and intrathecal therapy on the same day.(6) The risks associated with oral chemotherapy have also become better appreciated, especially as its use has become more common. Poor adherence, which reduces the chances of successful treatment, has been reported among selected populations with rates of 16% to 100%.(7) Common errors with oral chemotherapy involve dosing mishaps, sound-alike drug names, and supplying the wrong number of pills relative to the number of treatment days.(8) Taking a combination of pills of different strengths with interrupted daily or weekly treatment cycles can lead to confusion at home. Proposed solutions include the adoption of safe prescribing standards, a more meticulous approach to ensuring that patients and families are educated about the use and safe handling of these drugs, and developing better approaches for supporting adherence.(9)
In sum, there is limited data about the extent and nature of chemotherapy administration errors. The few published studies to date suggest that error rates are low, although these studies were performed in a small number of clinical settings. It is worth considering why chemotherapy error rates may be lower than error rates seen with other medications. Perhaps this reflects oncology clinicians' preoccupation with safe care, given the vulnerability of the patient population and the inherent toxicity of chemotherapy. While cancer patients generally perceive chemotherapy care to be safe, they recognize that potentially serious harm could result from a mistake.(10) As illustrated in the case, another protective factor may be that many cancer patients are acute observers of their own care and able to partner with providers to identify and intercept errors before they cause harm.
Case & Commentary—Part 2
On formal review of the case, it was determined that the outpatient oncologist (a specialist in penile and germ cell cancers) had recommended the appropriate 3-day regimen to the oncology fellow. In this medical center, there was a functioning electronic health record (EHR) and computerized provider order entry (CPOE) system, but the chemotherapy order sets still existed on paper. In choosing the chemotherapy regimen, the oncology fellow inadvertently chose the wrong paper order set—he saw that the order set included the correct agents but failed to notice the higher dose and incorrect duration. The inpatient attending oncologist, who had not previously met the patient and was less familiar with penile cancer, co-signed the fellow's incorrect orders. Throughout the hospitalization, the primary internal medicine team copied and pasted the original oncology outpatient note that stated the patient would receive the 3-day course of chemotherapy, even though this differed from the 5-day regimen that was ordered. None of the other safety checks that existed (including the presence of a chemotherapy pharmacist and chemotherapy nurse checking the orders against allergies, renal function, body surface area, etc.) identified the dose and duration error.
In 2009, the American Society of Clinical Oncology (ASCO) and the American Nursing Society co-published a comprehensive set of chemotherapy safety standards.(11,12) Chemotherapy administration was divided into seven discrete steps: review of clinical information and selection of a regimen; treatment planning and informed consent; ordering or prescribing; drug preparation; assessing treatment compliance; administration and monitoring; and response and toxicity monitoring (Table). Although the original focus was on outpatient administration of chemotherapy, it was soon recognized that patients receiving inpatient chemotherapy may be at increased risk of errors. In 2012, reflecting this concern, the standards were revised and updated with particular focus on the inpatient setting.(13,14)
The current case illustrates the potential risks of inpatient chemotherapy. A national trend, in place for more than a decade, has resulted in a shift in the setting in which chemotherapy is delivered.(15) Chemotherapy administration has gradually shifted to the outpatient setting based on growing expertise in chemotherapy administration, better management of adverse effects, availability of highly effective antiemetics, less toxic chemotherapeutic agents, and technologic advances allowing for ambulatory delivery of continuous infusions. Many regimens, including each of the agents used in this case, can be safely administered in the outpatient setting. Although the trend also reflects patient preference to avoid hospitalizations, it has come at a cost. Many hospitals have experienced a disintegration of their inpatient oncology services, with fewer dedicated nurses and pharmacists available to manage patients who require chemotherapy.(15) Dedicated oncology units staffed by medical oncologists have been replaced by teams of general internal medicine–trained hospitalist services. The lack of expertise and specialized experience certainly can increase the likelihood of errors. In addition, while most hospitals and clinics have or will soon implement sophisticated electronic health record and computerized provider order entry systems, the introduction of electronic chemotherapy order entry systems often lags behind the larger systems. Many versions of hybrid systems can result, for example, in some hospitals all medication orders except chemotherapy being electronic; in others, ambulatory chemotherapy is ordered using an electronic system, but inpatient chemotherapy is ordered using paper-based processes.
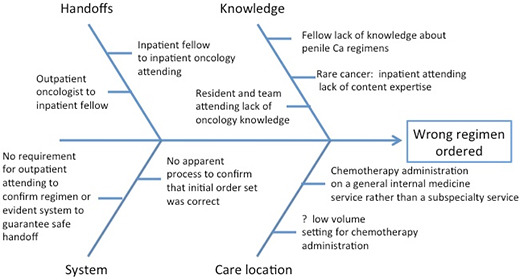
As is often discovered during a root cause analysis, the error in the current case reflected a series of process failures that resulted in ordering the wrong chemotherapy regimen.(16) Fortunately, an alert patient had the courage to speak up in time to mitigate the error. The initial error—pulling the wrong paper order set—went undetected by the outpatient oncologist, the inpatient attending (who co-signed the incorrect order set), the inpatient chemotherapy nurse, and the chemotherapy pharmacist. The Figure is an example of an Ishikawa Diagram that identifies major categories that contributed to the failure; it is based only on the data provided in this case (in practice, an Ishikawa Diagram is created during a root cause analysis in which those involved in the error identify all potential contributing factors). The failure to recognize that the wrong chemotherapy was ordered was likely a combination of several factors including multiple handoffs, lack of content expertise by the inpatient fellow and inpatient oncology attending, insufficient supervision by the attending oncologist, and the location of the patient on a non-oncology unit. Finally, organizations that oversee inpatient care, such as The Joint Commission, have not specifically focused attention on the risks of chemotherapy administration. As a result, hospitals may not have invested the same financial and personnel resources to prevent errors in this arena that they may have spent in others.
The single most important step the hospital should take to prevent further inpatient chemotherapy administration errors is to understand the current process for ordering and administering inpatient chemotherapy. As is true for many health care–associated practices, it is likely that the process is unstructured, highly variable, and unreliable. Based on a reliability scale created by the Institute for Healthcare Improvement (IHI), processes that lack clear structures and protocols tend to rely on vigilance and hard work to prevent errors. Experts have described that such unstructured approaches at best achieve 10-1 reliability, a level at which 1–2 defects/10 opportunities is expected.(17) This would imply that if 10 patients a day at a given hospital are receiving chemotherapy, 1–2 per day may experience a medical error.
The first step toward improving the reliability of inpatient chemotherapy administration for this practice would be to standardize the process. This could entail a structured handoff between the outpatient and inpatient providers, a checklist to be shared by the inpatient oncologists, nurses, and pharmacists to ensure proper communication, and structured documentation. For example, a synoptic chemotherapy treatment plan has been advocated by the ASCO as a structured communication tool.(18) To achieve 10-2 reliability (≤5 defects/100 opportunities) requires attention to human factors that contribute to failure in addition specific tools of improvement science. For example, a hierarchical organizational structure that discourages an employee from communicating concern can be improved by team training.(19) Finally, since the error also arose in part from a knowledge gap, it is not clear from the case that an electronic order entry system would have prevented the error.
The discovery of the error by the patient is notable and the team is to be commended for responding rapidly to prevent additional harm. This "intervention of last resort," though, cannot be depended upon. Not all patients are attentive, knowledgeable, and brave enough to voice concerns about their care.(20)
Take-Home Points
- In many hospitals, chemotherapy administration in the inpatient setting has become a "high-risk, low volume" procedure in which the risk of failure is high.
- The chemotherapy error occurred due to the lack of a high reliability framework and an over-reliance on vigilance and thoroughness.
- An electronic order entry system would not necessarily have prevented the error. Electronic solutions alone cannot overcome process failures.
Joseph O. Jacobson, MD, MSc
Chief Quality Officer, Dana Farber Cancer Institute
Associate Clinical Professor of Medicine, Harvard Medical School
Saul N. Weingart, MD, PhD
Vice President for Quality Improvement and Patient Safety, Dana Farber Cancer Institute
Associate Professor of Medicine, Harvard Medical School
Faculty Disclosure: Drs. Jacobson and Weingart have declared that neither they, nor any immediate member of their families, have a financial arrangement or other relationship with the manufacturers of any commercial products discussed in this continuing medical education activity. In addition, the commentary does not include information regarding investigational or off-label use of pharmaceutical products or medical devices.
References
1. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients—results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377-384. [go to PubMed]
2. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38:261-271. [go to PubMed]
3. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274:29-34. [go to PubMed]
4. Gandhi TK, Bartel SB, Shulman LN, et al. Medication safety in the ambulatory chemotherapy setting. Cancer. 2005;104:2477-2483. [go to PubMed]
5. Walsh KE, Dodd KS, Seetharaman K, et al. Medication errors among adults and children with cancer in the outpatient setting. J Clin Oncol. 2009;27:891-896. [go to PubMed]
6. Hennipman B, de Vries E, Bökkerink JPM, Ball LM, Veerman AJP. Intrathecal vincristine: 3 fatal cases and a review of the literature. J Pediatr Hematol Oncol. 2009;31:816-819. [go to PubMed]
7. Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652-661. [go to PubMed]
8. Weingart SN, Toro J, Spencer J, et al. Medication errors involving oral chemotherapy. Cancer. 2010;116:2455-2464. [go to PubMed]
9. Neuss MN, Polovich M, McNiff K, et al. 2013 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards including standards for the safe administration and management of oral chemotherapy. J Oncol Pract. 2013;9:5s-13s. [Available at]
10. Schwappach DL, Wernli M. Chemotherapy patients' perceptions of drug administration safety. J Clin Oncol. 2010;28:2896-2901. [go to PubMed]
11. Jacobson JO, Polovich M, McNiff KK, et al. American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards. J Clin Oncol. 2009;27:5469-5475. [go to PubMed]
12. Jacobson JO, Polovich M, McNiff KK, et al. American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards. Oncol Nurs Forum. 2009;36:651-658. [go to PubMed]
13. Jacobson JO, Mulvey TM. Time to focus on inpatient safety: revision of the American Society Of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards. J Clin Oncol. 2012;30:1021-1022. [go to PubMed]
14. Jacobson JO, Polovich M, Gilmore TR, et al. Revisions to the 2009 American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards: expanding the scope to include inpatient settings. J Oncol Pract. 2012;8:2-6. [go to PubMed]
15. Williamson TS. The shift of oncology inpatient care to outpatient care: the challenge of retaining expert oncology nurses. Clin J Oncol Nurs. 2008;12:186-189. [go to PubMed]
16. Reason JT, Carthey J, de Leval MR. Diagnosing "vulnerable system syndrome": an essential prerequisite to effective risk management. Qual Health Care. 2001;10(2 suppl):ii21-ii25. [go to PubMed]
17. Resar RK. Making noncatastrophic health care processes reliable: learning to walk before running in creating high-reliability organizations. Health Serv Res. 2006;41(4 Pt 2):1677-1689. [go to PubMed]
18. Developing the medical oncology treatment plan and summary. J Oncol Pract. 2006;2:95-96. [go to PubMed]
19. Bunnell CA, Gross AH, Weingart SN, et al. High performance teamwork training and systems redesign in outpatient oncology. BMJ Qual Saf. 2013;22:405-413. [go to PubMed]
20. Schwappach DL, Wernli M. Barriers and facilitators to chemotherapy patients' engagement in medical error prevention. Ann Oncol. 2011;22:424-430. [go to PubMed]
Table
Table. American Society of Clinical Oncology (ASCO) and the American Nursing Society Chemotherapy Safety Standards.| Chemotherapy administration comprises 7 steps: |
|---|
| 1) Review of clinical information and selection of a regimen |
| 2) Treatment planning and informed consent |
| 3) Ordering or prescribing |
| 4) Drug preparation |
| 5) Assessing treatment compliance |
| 6) Administration and monitoring |
| 7) Response and toxicity monitoring |
Figure
Figure. Example of an Ishikawa Diagram that identifies major categories that contributed to the failure in this case.