The Worst Headache
The Case
A 48-year-old woman with a history of migraine headaches and hypertension presented to her outpatient clinic with a 4-day history of headache. While shopping 4 days earlier, she experienced sudden onset of a severe diffuse headache—"maybe the worst headache I've ever had." She sat down because of the pain and associated nausea.
She had presented to clinic later that day, where a nurse practitioner assessed her symptoms as consistent with her prior migraines, and recommended that she simply start the regimen that she had used in the past (ibuprofen and ergotamine tartrate/caffeine [Cafergot®]).
When her symptoms remained severe, she returned the following day to the urgent care center. A staff physician agreed with the nurse's diagnosis and reassured the patient that there simply had not been enough time for the medications to take effect. He administered intramuscular ketorolac and oral prochlorperazine, with substantial improvement in her symptoms. An appointment was made for her to follow-up with her primary care physician 3 days later in case symptoms persisted, and also to discuss initiation of a medication for migraine prophylaxis.
When the patient returned for her clinic visit in the late afternoon 3 days later, she initially stated that her symptoms had resolved. On closer questioning, however, she stated that she continued to experience headaches when straining (eg, during bowel movements) or bending over. Her physical exam, including visualization of both retinas, was normal.
The physician regarded the initial acute presentation as very worrisome for subarachnoid hemorrhage (SAH). However, her subsequent clinical course seemed too benign, even with the lingering headaches. Given that he had not completely ruled out the possibility of hemorrhage, he arranged for her to have a CT scan and asked the radiologist to page him immediately with the results. He also gave the patient clear instructions to call him if her symptoms worsened.
The radiologist paged the primary physician later that evening to inform him that the head CT was normal. Knowing that the CT is not 100% sensitive for subarachnoid hemorrhage, the physician telephoned the patient the next morning to see how she was doing. She had just woken up, but thanked him for calling and stated that she felt much better—then the phone went dead. At first, the physician thought she had simply hung up, but since it was rather abrupt he called back and received a busy signal. He called 911.
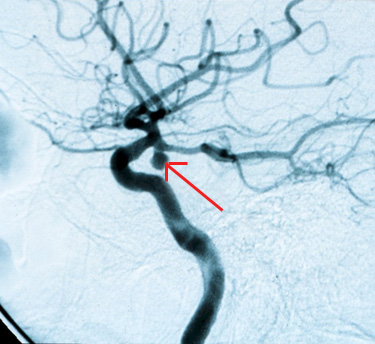
EMTs found the patient on the floor, arousable only to painful stimuli. MR angiography in the emergency department demonstrated a posterior circulation aneurysm (Figure 1), which was clipped later that day. The patient required a ventriculoperitoneal shunt, but her postoperative course went well, with complete neurologic recovery.
The Commentary
Even in this age of ready access to sophisticated neuro-imaging modalities, misdiagnosis of patients with non-traumatic subarachnoid hemorrhage occurs in roughly 20%-30% of cases (1-3) and results in an inordinate number of malpractice claims.(4) Why is this? Three recurring explanations may account for this high rate of misdiagnosis: (i) not considering the diagnosis, (ii) not understanding the limitations of computed tomography (CT), and (iii) not performing or correctly interpreting the results of lumbar puncture (LP).
Failure to Consider the Diagnosis In the ambulatory setting, headache accounts for about 4% of office visits and 2% of emergency department (ED) visits.(1,2) Even in the acuity-skewed ED population, less than 1% of headache patients have SAH, and only an additional 3%-4% have some other serious condition that is life, limb, vision, or brain threatening if untreated (Table 1). The clinician's principal goal is to distinguish between the 95% of patients with self-limiting headache syndromes, who require pain control, and the other 5%, who require emergent diagnosis and treatment.
The clinician's task may seem sufficiently similar to finding a needle in a haystack that high rates of misdiagnosis ought to be expected. However, clinicians operate with a number of misconceptions about patients with headaches in general and SAH in particular. First, because migraine and tension headache are so common, patients with a history of headaches sometimes develop other types of headache, as occurred in the present case. Since almost everyone has had a headache at some point, clinicians must carefully distinguish the index headache from prior ones.
We are not told how the nurse practitioner "assessed her symptoms as consistent with her prior migraines." A simple battery of questions will quickly distinguish "familiar" or chronic headaches from unusual ones. In general, relevant questions include:
- Are the quality, duration, severity, and onset of pain the same?
- Are any associated symptoms the same?
- How often and under what circumstances have headaches occurred in the past?
- Has there been any kind of work-up for the headaches?
- Is the current headache responding to treatment as in the past?
Many clinicians have the mistaken impression that patients with SAH invariably exhibit at least one of the following: severe, acute illness; hard neurological findings such as cranial nerve palsies; or meningismus. This is simply not true. As with most diagnoses in medicine, there is a bell-shaped curve of presentations, and well-appearing patients with normal neurological examinations represent approximately 30%-40% of patients with SAH.(1) Reliance on the classic presentation of any disease opens the door to misdiagnosis. Moreover, these mildly affected patients, who may lack classic symptoms and signs, have the most to gain by prompt correct diagnosis and therapy.
Another misconception is that symptoms begin with strenuous activity. In fact, in one large study, roughly 50% of patients with SAH developed their initial symptoms during relaxed activity or were awakened from sleep.(5) Similarly, many patients will not describe their pain as "10 out of 10" in severity or characterize it as the "worst headache of my life." Moreover, some patients report their symptoms as gradual in onset, rather than describing a "thunderclap" (sudden) onset headache. However, almost all patients will describe their headache as unusual or distinctive in terms of severity and/or character. Even when neck pain dominates the clinical picture, it too tends to be unusual and distinctive, reinforcing the importance of carefully characterizing the patient's symptoms, especially when there is a prior history of headache. Table 2 lists common misdiagnoses given to SAH patients.
Another common misconception is that reduction of pain with analgesics excludes severe intracranial pathology. Pathophysiologically, a limited number of pathways mediate head pain. Since medications of any class (ie, non-steroidals, opiate analgesics) may blunt or eradicate headache, no diagnostic information should be ascribed to this response. Adding further to the challenge, the pain from a small SAH can resolve spontaneously.
With regard to history, there are two other important and related factors, at least one of which may have played a role in this case. The first is that patients will often use the words "migraine" and "sinus-headache" imprecisely. Some use "migraine" to mean any severe headache, whereas in fact, it has particular characteristics that are well-defined by the International Headache Society.(6) Therefore, if a patient says "I had two migraines last week," the clinician should proceed as if the patient used the word "headache" until more questions confirm the headaches are indeed migraines.
The second phenomenon is that of "anchoring." The patient in this case is diagnosed (incorrectly) with migraine, which then becomes part of her (recent) past history. Psychologically, this limits the open-mindedness of subsequent clinicians evaluating the same problem. Remember that definitive diagnosis of either tension or migraine headache requires multiple episodes of typical symptoms (5 for migraine, 10 for tension headache). Therefore, one should be very cautious of making these diagnoses in patients presenting with their first or worst headache.
Physical examination in patients with SAH is often normal. Only 10%-15% of patients exhibit a third-nerve palsy—85% of aneurysms are located in the anterior circulation and are therefore unlikely to produce any cranial nerve findings.(2) Meningismus and ocular hemorrhages also occur only in a minority of patients.
For all these reasons, not considering SAH in the differential diagnosis remains the most common reason for misdiagnosis.(1,3) Failure to consider the diagnosis commonly results in failure to obtain relevant investigations—specifically, computed tomography and lumbar puncture. Even when these tests are performed, however, clinicians sometimes fail to interpret the results correctly, prematurely terminating the work-up in the face of an initial negative result.
The Limitations of CT Scanning CT is a powerful diagnostic tool in SAH, but has important limitations. Putting aside misinterpretation, the two most serious issues are spectrum bias and timing from onset of headache.(1,2) In spectrum bias, less severely affected patients (on the left side of the bell-shaped curve of severity) have smaller bleeds and therefore are more likely to have a negative CT scan.(7,8) Timing is also crucial. During the first 12 hours after onset of the headache, CT sensitivity approaches 98%. However, the 95% confidence intervals around this number are wide, and therefore most still recommend a negative CT be followed by a lumbar puncture (LP).(8,9) Three days after onset of the headache, the CT is only 85% sensitive, and at 7 days, it is only 50% sensitive.(10)
In this case, the patient's CT was on the fourth day after onset of headache. The negative study at this stage (which would have a sensitivity of about 80%) should not have reassured the clinician and the correct next step would have been to perform an LP. Because this is often viewed as overly aggressive in these well-appearing patients, clinicians often forego this step.(11)
Interpreting the LP Results The LP in patients with SAH may show blood, xanthochromia, or elevated opening pressure. Blood can be from a traumatic tap or from true SAH; there are many methods of distinguishing between the two, although none is entirely foolproof.(12) Xanthochromia is probably the best method (Figure 2). Many believe that xanthochromia must be measured by spectrophotometry; however this technique is not used in 99% of hospital laboratories in North America.(13) In addition, it may be overly sensitive, with many false positives.(11) Experimentally, CSF with red blood cell counts above 10,000 RBCs/µL or samples that have sat for a period of time prior to analysis may show xanthochromia in the absence of SAH.(14) An elevated opening pressure is found in roughly two thirds of patients with SAH and will occasionally suggest the alternative diagnoses of idiopathic intracranial hypertension and cerebral venous thrombosis.(1)
The best way to avoid misdiagnosis of patients with SAH is to take a very detailed history of the current headache, and to compare it with prior headaches. In patients with abnormal physical examinations, the decision to further evaluate the patient with CT, followed by LP if the CT is negative, equivocal or technically inadequate, is clear-cut. In patients with normal exams, clinicians should stay open to the possibility of SAH and have a very low threshold for pursuing the same evaluation (CT followed by LP) if this life-threatening disorder remains in the differential diagnosis based on the history.
Jonathan A. Edlow, MD Associate Chief, Department of Emergency Medicine, Beth Israel Deaconess Medical Center Assistant Professor of Medicine, Harvard Medical School
References
1. Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000;342:29-36.[ go to PubMed ]
2. Edlow JA. Diagnosis of subarachnoid hemorrhage in the emergency department. Emerg Med Clin North Am. 2003;21:73-87.[ go to PubMed ]
3. Kowalski RG, Claassen J, Kreiter KT, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA. 2004;291:866-9.[ go to PubMed ]
4. Karcz A, Holbrook J, Burke M, et al. Massachusetts emergency medicine closed malpractice claims: 1988-1990. Ann Emerg Med. 1993;22:553-9.[ go to PubMed ]
5. Schievink WI, Karemaker JM, Hageman LM, van der Werf DJ. Circumstances surrounding aneurysmal subarachnoid hemorrhage. Surg Neurol. 1989;32:266-72.[ go to PubMed ]
6. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia. 1988;8 Suppl 7:1-96.[ go to PubMed ]
7. Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The international cooperative study on the timing of aneurysm surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18-36.[ go to PubMed ]
8. van der Wee N, Rinkel GJ, Hasan D, van Gijn J. Detection of subarachnoid haemorrhage on early CT: is lumbar puncture still needed after a negative scan? J Neurol Neurosurg Psychiatry. 1995;58:357-9.[ go to PubMed ]
9. Edlow JA, Wyer PC. Evidence-based emergency medicine/clinical question. How good is a negative cranial computed tomographic scan result in excluding subarachnoid hemorrhage? Ann Emerg Med. 2000;36:507-16.[ go to PubMed ]
10. van Gijn J, van Dongen K. The time course of aneurysmal haemorrhage on computed tomograms. Neuroradiology. 1982;23:153-6.[ go to PubMed ]
11. Morgenstern LB, Luna-Gonzales H, Huber JC Jr, et al. Worst headache and subarachnoid hemorrhage: prospective, modern computed tomography and spinal fluid analysis. Ann Emerg Med. 1998;32:297-304.[ go to PubMed ]
12. Shah KH, Edlow JA. Distinguishing traumatic lumbar puncture from true subarachnoid hemorrhage. J Emerg Med. 2002;23:67-74.[ go to PubMed ]
13. Edlow JA, Bruner KS, Horowitz GL. Xanthochromia. Arch Pathol Lab Med. 2002;126:413-15.[ go to PubMed ]
14. Graves P, Sidman R. Xanthochromia is not pathognomonic for subarachnoid hemorrhage. Acad Emerg Med. 2004;11:131-5.[ go to PubMed ]
Tables
Table 1. "Cannot Miss" Causes of Headache. [Diseases or conditions that are both treatable and, if untreated, are life, limb, brain, or vision threatening.]
| Subarachnoid hemorrhage |
| Meningitis and encephalitis |
| Cervico-cranial artery dissections |
| Temporal arteritis |
| Acute narrow angle closure glaucoma |
| Hypertensive emergencies |
| Carbon monoxide poisoning |
| Pseudotumor cerebri |
| Cerebral venous and dural sinus thrombosis |
| Acute strokes: hemorrhagic or ischemic |
| Pituitary apoplexy |
| Mass lesions |
| Tumor |
| Abscess |
| Intracranial hematomas (parenchymal, subdural, epidural) |
| Parameningeal infections |
| Colloid cyst of 3rd ventricle |
Table 2. Incorrect Diagnoses Assigned to Patients with SAH
| No diagnosis made, or headache of unknown etiology |
| Primary headache disorders: migraine, tension, or cluster headaches |
| Meningitis and encephalitis |
| Systemic infection: flu, gastroenteritis, viral syndrome |
| Stroke or cerebral ischemia |
| Hypertensive crisis |
| Cardiovascular diagnosis: rule out MI, arrhythmia, or syncope |
| Sinus-related headache |
| Neck problems: cervical disc disease or arthritis |
| Psychiatric diagnosis: including malingering and alcohol intoxication |
| Trauma-related |
| Back pain |
Figures
Figure 1. Regular Angiography of Posterior Communicating Artery Aneurysm (arrow).
Figure 2. One Tube of Xanthochromic Fluid (left) Compared to Normal CSF.