CA-MRSA Skin Infections: An Ounce of Prevention is Worth a Pound of Cure
Liu C. CA-MRSA Skin Infections: An Ounce of Prevention is Worth a Pound of Cure. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2012.
Liu C. CA-MRSA Skin Infections: An Ounce of Prevention is Worth a Pound of Cure. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2012.
Case Objectives
- Identify risk factors for transmission of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infection.
- Describe the primary management of a cutaneous abscess.
- Describe steps schools, coaches, and athletes can take to prevent infection.
- Describe steps schools, coaches, and athletes can take to promote early recognition of infection.
The Case
A 16-year-old adolescent boy was a member of his junior varsity wrestling team. One morning during wrestling season, he noticed a sore spot on his left buttock, assumed it was an insect bite, and applied hydrocortisone cream. Two days later, he noticed the bump was larger with a small amount of pus at the center. Assuming it was an ingrown hair, he expressed the pus. He attended school and practice the next day and did not mention the bump to his parents, teachers, or coach. It was his turn for "mat duty" after practice, and he used the same mop that was used every day to wipe down the mats. As the week progressed, he noticed the bump was increasingly tender and painful to sit on. After several days, the lesion had grown larger and was now red, raised, and warm, and he notified his mother.
His mother took him to the pediatrician that day. His vital signs were within normal range and he rated his pain 6/10. His pediatrician, who had seen him regularly since birth, noted that his immunizations were up to date and he had no chronic illnesses or prior hospitalizations. He had a history of a Septra allergy (trimethoprim-sulfamethoxazole), with a rash associated with past treatment of otitis media. The pediatrician ruled out brown recluse spider bite due to geography, and his wrestling made the pediatrician suspicious of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) skin infection. The pediatrician incised the lesion, expressed pus, and cultured the fluid. Considering his Septra allergy, the pediatrician prescribed oral clindamycin 450 mg every 6 hours for 10 days, with follow-up in 7 days, and ibuprofen for pain.
Four days after starting antibiotics, the patient complained of hip pain with ambulation and he assumed he had pulled something during wrestling practice. He was observed to be pale and complained of chills and hip pain. His temperature was 102° F. His mother noted the lesion was larger and his whole buttock was red and swollen with red streaks. She immediately took her son to the emergency department (ED).
Deep tissue debridement of the hip lesion was performed in the intensive care unit (ICU) because the patient was not stable enough to have it performed in the operating room. Despite the debridement and appropriate antibiotics, by hospital day 3, he developed acute renal failure necessitating peritoneal dialysis and respiratory failure requiring intubation. Results from the original wound culture revealed CA-MRSA resistant to clindamycin. A radiograph showed extensive erosion at the femur head secondary to osteomyelitis.
The patient had a prolonged and stormy hospital course. On ICU day 20 he was extubated and moved to the step-down unit. As a result of the femur head destruction, he required a left total hip replacement. He continued physical therapy at home and was referred to a psychologist for long-term management of the issues surrounding his disability. It is expected that he will require hip replacements every 10 to 30 years for the rest of his life.
The Commentary
This otherwise healthy young man presented initially with a pustule on his left buttock, which progressed to a larger abscess for which he appropriately received incision and drainage. Unfortunately, the patient developed a complication of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infection: progression of his soft tissue infection to femoral head osteomyelitis requiring total hip replacement. This case illustrates potential opportunities for patient and school/athletic team education toward prevention and recognition of this disease as well as some of the challenges in management of CA-MRSA skin and soft tissue infections (SSTIs).
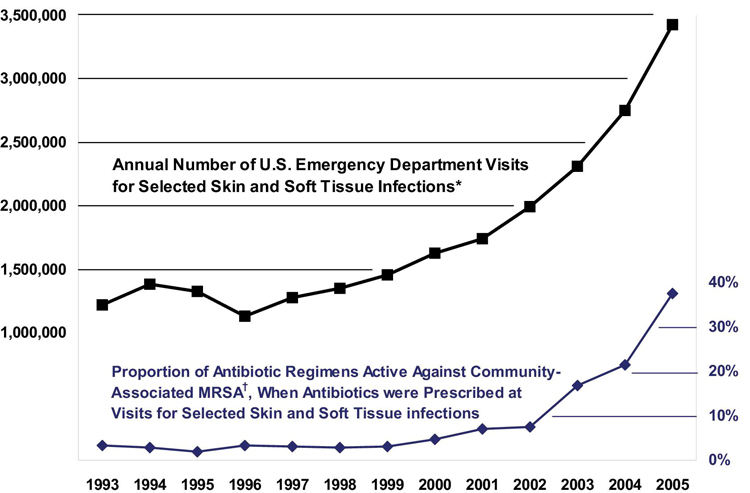
Over the last decade, CA-MRSA has emerged as the most common cause of SSTIs in the United States. Initially almost exclusively health care–associated, by the mid-1990s, MRSA strains were reported to cause infections among previously healthy individuals in the community who lacked the usual health care–associated risk factors. CA-MRSA is genetically distinct from health care–associated MRSA (HA-MRSA) and is often susceptible to many non–β-lactam antibiotics (e.g., doxycycline, clindamycin). In a study of patients presenting with purulent SSTIs to 11 emergency departments (EDs) throughout the US in August 2004, MRSA was identified in 59% of the cases (1); when the study was repeated in 2008, the same prevalence was observed.(2) ED visits for SSTI increased almost threefold from 1993 to 2005 (Figure 1), from 1.2 million visits (1.35% of all ED visits) to 3.4 million visits (2.98% of all ED visits), concurrent with the widespread emergence of CA-MRSA.(3) The National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey from 1997 to 2005 demonstrated that ambulatory visits for abscess/cellulitis nearly doubled, suggesting that this epidemic has had a major impact on office-based practice (4), as illustrated by this case.
Outbreaks of CA-MRSA have been reported in a variety of patient populations including prison inmates, military personnel, urban underserved communities, indigenous populations, children attending daycare centers, and athletes.(5) In a survey of 312 high schools in Nebraska, the prevalence of physician-confirmed MRSA infections in athletes increased from 4.4% to 14.4% between 2006–2007 and 2007–2008. During the same timeframe, the incidence of MRSA per 10,000 wrestlers increased from 19.6 to 60.1.(6) Common exposures shared by these groups that may facilitate transmission include poor hygiene, crowded living conditions or other close contact settings, and direct or indirect contact (e.g., via shared personal items) with an active carrier or case.(6,7) The Centers for Disease Control and Prevention (CDC) uses the 5 C's to describe risk factors for transmission of CA-MRSA infection: Crowding, Contact (frequent skin-to-skin), Compromised skin (e.g., cuts and abrasions), Contaminated items and surfaces, and lack of Cleanliness. Low socioeconomic status has also been identified as a common link among some of the high-risk groups, although a number of patients with CA-MRSA have no obvious risk factors.(6)
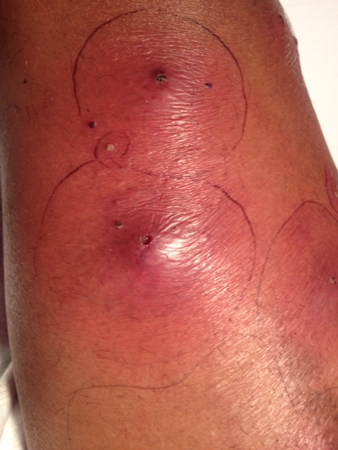
The patient's pediatrician appropriately recognized that this patient had an abscess and maintained a high index of suspicion for CA-MRSA infection. Among athletes, CA-MRSA outbreaks have occurred at the high school, college, and professional level for football, rugby, and wrestling.(5,8) In each of these cases, the primary site of infection was cutaneous with the major modes of transmission being direct skin-to-skin contact or common-source exposure (e.g., shared soap, towels, razors, athletic training equipment).(9-11) Furthermore, skin trauma that occurs during contact sports likely facilitates acquisition of infection after exposure.(8) It is notable that the patient ascribed his infection to an insect bite. Interestingly, studies have found that the presence of lesions initially falsely attributed to spider bites was predictive of having an infection due to CA-MRSA.(1,12) In one study of ED patients reporting a spider bite, the vast majority were diagnosed with an SSTI with only 3.8% having actual spider bites.(12) In some cases, these lesions may have a central eschar or dermonecrotic center, resembling the bite of a brown recluse spider, although the geographic distribution of this spider should exclude this possibility in non-endemic areas. Thus, clinicians should consider the possibility of MRSA infection in patients who report spider bites (Figure 2). Concurrent infection of actual spider bites is very rare.
The most critical step in the management of any cutaneous abscess is incision and drainage, as was performed by his pediatrician. As the patient had normal vital signs without systemic signs or symptoms, it would have been appropriate and consistent with the 2011 Infectious Diseases Society of America (IDSA) MRSA guidelines to not treat with adjunctive antibiotic therapy.(13) In contrast with cellulitis, in which case antibiotics are essential, cutaneous abscesses without cellulitis may not require antibiotics. Multiple observational studies have shown high cure rates (85%–90%) whether or not an active antibiotic is used; randomized clinical trials showed no significant difference in the primary outcome of clinical cure whether patients received antibiotic or placebo.(14-16) The IDSA MRSA guidelines state, "For simple abscesses or boils, incision and drainage alone is likely adequate but additional data are needed to further define the role of antibiotics, if any, in this setting."(13) At this point, it remains unknown whether antibiotics provide any clinically significant benefit beyond clinical cure (e.g., prevention of recurrent infections or progression to deep tissue infection or more complicated disease). Based on currently available published literature and data presented in this case study, it is unclear that the use of clindamycin, to which the organism was not susceptible, led to progression of this patient's disease (versus inadequate initial drainage or other factors). The vast majority of patients with simple cutaneous abscesses resolve their infection with incision and drainage; unfortunately, this patient represents the very rare case where complications and deeper infection occur. Further research is needed to identify risk factors for progression to invasive disease.
That said, steps could have been taken that might have helped this patient. Instead of waiting 1 week for follow-up, the patient should have been scheduled for a follow-up appointment 2 to 3 days after the procedure and provided instructions to return sooner with worsening signs or symptoms.(17) Early follow-up might have resulted in earlier recognition of disease progression and determination of the need for additional incision and drainage. In addition, an early follow-up visit would have provided an opportunity to review culture results and susceptibilities and to modify antibiotic therapy accordingly. The IDSA guidelines recommend use of adjunctive antibiotic therapy after incision and drainage in cases where the patient fails to respond to incision and drainage alone and where there is rapidly progressive disease in the presence of associated cellulitis.(13)
This case also illustrates multiple opportunities to improve infection prevention and recognition. Good personal and environmental hygiene are key strategies to infection prevention.(11,18) Athletes should clean hands with soap and water or use an alcohol-based hand rub before and after practice and using shared weight-training equipment. They should be advised to take care of their skin by wearing protective clothing or gear and to cover any skin abrasions or cuts with clean dry bandages or dressings. They should avoid sharing personal items that come into contact with bare skin such as towels, razors, and bar soaps and use a barrier (e.g., towel) between skin and shared equipment. Showering is recommended immediately after exercise, particularly after activities with direct skin contact with people or shared equipment/surfaces. School sports team administrators and coaches should provide facilities and equipment necessary to promote good hygiene, such as adequate supplies of soap and towels, and maintain clean facilities as well as access to cleaning supplies used after practice. In this case, a dirty mop was what was made available to clean the mats. Coaches and parents should encourage good hygiene among athletes, be taught to administer proper first aid, and role model appropriate hand hygiene.
To improve early infection recognition, athletes should receive basic education regarding signs and symptoms of skin infection. Athletes should be strongly encouraged to inform their parents, coach, school nurse, or team doctor of potential infection to expedite evaluation and treatment. Skin infections have been associated with 17% practice-time loss injuries in wrestling.(19) In this case, the patient participated in practice with an active infection without telling anyone, which led to a delay in treatment and potentially placed other team members at risk for disease transmission. A system should be established to identify athletes with skin wounds and infections prior to play to ensure that wounds are appropriately covered and those with active infections are excluded and receive prompt treatment. The National Collegiate Athletic Association (NCAA) Wrestling Rules and Interpretations recommend that visual examination of the skin be conducted by physicians and/or certified athletic trainers prior to all meets and tournaments. Wrestlers with an SSTI must be excluded from play until at least 72 hours after initiation of therapy and be allowed to participate only after there is marked clinical improvement and no new lesions appearing for 48 hours. In addition, an intact occlusive dressing must be in place before and during competition.(19)
CA-MRSA remains a dominant cause of SSTI and can lead to devastating consequences in young athletes, in some cases sidelining entire careers, such as in this tragic case. A proactive approach toward infection prevention will avoid development of a problem in the first place, and early recognition of infections will minimize the likelihood of long-term morbidity. All individuals associated with competitive sports teams, including players, coaches, teachers, parents, and administrators, can help prevent sports-related skin infections.
Take-Home Points
To prevent sports-related skin infections, everyone involved in sports teams should:
- Encourage good hygiene among athletes, including showering and washing with soap after all practices and competitions. Avoid sharing personal items such as bar soaps, towels, and razors.
- Establish routine cleaning schedule of shared equipment; ensure availability of adequate cleaning supplies.
- Encourage athletes to report skin lesions to coaches and develop system for coaches to assess athletes for skin lesions before practices and competitions.
- Train athletes and coaches in first aid and recognition of potentially infected wounds.
- Cover all wounds. If a wound cannot be covered adequately or is infected, exclude athlete from play until the wound can be appropriately covered, and in the case of infection, until lesions are healed or significantly improved.
Catherine Liu, MD
Assistant Clinical Professor
School of Medicine
University of California, San Francisco
Faculty Disclosure: Dr. Liu has declared that neither she, nor any immediate member of her family, has a financial arrangement nor other relationship with the manufacturers of any commercial products discussed in this continuing medical education activity. The commentary does not include information regarding investigational or off-label use of pharmaceutical products or medical devices.
References
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al; EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674. [go to PubMed]
2. Talan DA, Krishnadasan A, Gorwitz RJ, et al; EMERGEncy ID Net Study Group. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin Infect Dis. 2011;53:144-149. [go to PubMed]
3. Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA Jr. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2008;51:291-298. [go to PubMed]
4. Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med. 2008;168:1585-1591. [go to PubMed]
5. David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616-687. [go to PubMed]
6. Buss BF, Mueller SW, Theis M, Keyser A, Safranek TJ. Population-based estimates of methicillin-resistant Staphylococcus aureus (MRSA) infections among high school athletes—Nebraska, 2006–2008. J Sch Nurs. 2009;25:282-291. [go to PubMed]
7. Liu C, Graber CJ, Karr M, et al. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004–2005. Clin Infect Dis. 2008;46:1637-1646. [go to PubMed]
8. Turbeville SD, Cowan LD, Greenfield RA. Infectious disease outbreaks in competitive sports: a review of the literature. Am J Sports Med. 2006;34:1860-1865. [go to PubMed]
9. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352:468-475. [go to PubMed]
10. Lindenmayer JM, Schoenfeld S, O'Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med. 1998;158:895-899. [go to PubMed]
11. Centers for Disease Control and Prevention. Methicillin-resistant staphylococcus aureus infections among competitive sports participants—Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000–2003. MMWR Morb Mortal Wkly Rep. 2003;52:793-795. [go to PubMed]
12. Suchard JR. "Spider bite" lesions are usually diagnosed as skin and soft-tissue infections. J Emerg Med. 2011;41:473-481. [go to PubMed]
13. Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18-e55. [go to PubMed]
14. Duong M, Markwell S, Peter J, Barenkamp S. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med. 2010;55:401-407. [go to PubMed]
15. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048. [go to PubMed]
16. Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56:283-287. [go to PubMed]
17. Fitch MT, Manthey DE, McGinnis HD, Nicks BA, Pariyadath M. Videos in clinical medicine. Abscess incision and drainage. N Engl J Med. 2007;357:e20. [go to PubMed]
18. MRSA Infections. Prevention Information and Advice for Athletes. Centers for Disease Control and Prevention; 2010. [Available at]
19. Bubb RG. Skin Infections in Wrestling. Indianapolis, IN: National Collegiate Athletic Association; August 2009. 2010 and 2011 NCAA Wrestling Rules and Interpretations; Appendix B. [Available at]
Figures
Figure 1. Annual visits to US EDs for skin and soft tissue infections, during the emergence of community-associated MRSA, 1993-2005. Reprinted from (3) with permission from American College of Emergency Physicians.
Figure 2. Example of typical CA-MRSA lesions (multiple abscesses) that a patient attributed to a spider bite.