Preventing PICC Complications: Whose Line Is It?
Moureau N. Preventing PICC Complications: Whose Line Is It?. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2012.
Moureau N. Preventing PICC Complications: Whose Line Is It?. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2012.
The Case
A 55-year-old woman with myasthenia gravis, hypertension, and hypothyroidism presented to the emergency department with 1 week of progressive left arm swelling, neck pain, and fevers. For the past year, the patient was receiving treatment for myasthenia gravis with intravenous immunoglobulin (IVIG) through a peripherally inserted central catheter (PICC). On admission, she was found to have extensive catheter-related thrombosis in the subclavian, axillary, and internal jugular veins. Her blood cultures subsequently grew staphylococcus aureus and she was diagnosed with endocarditis and osteomyelitis of her cervical spine. Her hospital course was complicated by sepsis, acute respiratory distress syndrome (ARDS), and multiorgan failure. The patient ultimately died during the hospitalization.
The hospital's quality committee reviewed the case. They noted that the patient had a PICC line placed at one facility but was receiving IVIG infusions at a different hospital closer to home. Questions were raised about who had responsibility for the line, whether it should have been replaced periodically to reduce infection risk, and what other strategies might have prevented this outcome.
The Commentary
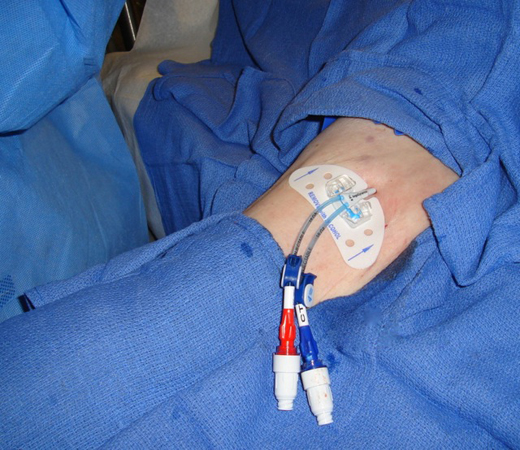
More than 2.5 million peripherally inserted central catheters (PICCs) (Figure) are placed in acute care facilities in the United States, and 5 million internationally, per year.(1,2) This rapid growth in PICC utilization has been fueled by ease of bedside placement and better technology, such as ultrasound and tip navigation. These techniques increase placement success, obviate the need for fluoroscopic guidance, and can be done safely at the bedside. Hospital-based PICC teams and individual PICC specialty services can increase the appropriate use of PICCs and reduce the time to placement. According to the Centers for Disease Control and Prevention (CDC), "specialized 'IV teams' have shown unequivocal effectiveness in reducing the incidence of CRBSI [catheter-related bloodstream infections], associated complications, and costs."(3) With the increased use of PICCs, clinicians and their patients now require a greater understanding of PICC-specific complications, especially where PICC teams may not exist. The case presented illustrates both the ability of PICCs to provide advanced therapies outside the hospital setting and the vigilance required in caring for the PICC itself to minimize the risk of complications.
PICC Placement and Management
In patients who require intravenous access, appropriate indications for PICCs include: (i) when there is difficulty maintaining peripheral access; (ii) for intravenous treatment longer than 5 days; (iii) for any infusions known to damage the intima of the vein such as irritants, vesicants, and chemotherapeutic agents; (iv) for infusions of total parenteral nutrition or other hyperosmolar solutions; (v) for patients with coagulopathies, respiratory issues, a tracheostomy, on mechanical ventilation, or other conditions of the chest or neck that increase risk of complications with use of other central venous catheter (CVC) access; and (vi) for patients needing prolonged outpatient treatment.
Once the PICC is placed, a plan should be initiated to provide for daily site assessment, routine flushing, sterile dressing changes, and medication administration. The patient in this particular case had varied services involved, from multiple organizations. The first hospital provided initial placement; the second hospital administered medications and likely, sterile dressing changes; and the patient probably received home management instructions for daily self-care. The primary responsibility for the PICC lies with the hospital/facility providing the regular treatment, which was the second hospital in this example. The hospital providing regular treatment services should perform functions of aseptic access, evaluation of the insertion site and device function, and daily assessment for complications and line necessity. Because the optimal time for a PICC to remain in place (dwell-time) is unknown and the CDC does not recommend catheter exchange for the purpose of infection prevention, maintaining a PICC for months or years is within the standard of practice.
PICC Complications and Prevention Strategies
Infection and thrombosis are the two most serious complications associated with PICCs or any other CVC. Because thrombosis can lead to infection, early identification and treatment of thrombosis reduces this risk.(4-6) Educating providers and patients about the signs and symptoms of thrombosis and infection is necessary to hasten identification and treatment, avoiding more significant morbidity.
Infection rates with PICCs are similar to or less than nontunneled CVCs.(7,8) Compliance with safe practices helps to promote a higher quality of patient care.(9) Groups such as the Institute for Healthcare Improvement (IHI) in conjunction with the CDC emphasize the use of maximum sterile barriers for all CVC insertions. Based on the landmark Keystone study (10) that took place within intensive care units in Michigan, five areas of practice (the "central line bundle") were stressed for CVC placement. The five bundle practices are (i) hand hygiene prior to the procedure; (ii) site selection for reduced insertion and infection risk; (iii) chlorhexidine skin disinfection prior to insertion; (iv) use of maximum sterile barriers throughout; and (v) daily evaluation for catheter necessity with prompt removal.(10) In a recent report of a patient safety project by the Agency for Healthcare Research and Quality (AHRQ), infections were reduced by 40% with application of the bundle through a comprehensive unit-based safety program (CUSP) customized for each institution.(11) Adoption of the bundle has led to remarkable reductions in CVC-related complications, both in Michigan and now nationally.
Current hospital-based education for physicians and nurses focuses on infection prevention, particularly the necessity of aseptic technique specific to intravenous insertion and care. Hand hygiene is stressed along with the need to thoroughly disinfect each access point prior to infusion through the intravenous tubing and vein. In the present case, the patient required intravenous immunoglobulin (IVIG) to treat her underlying neurologic condition. As an immunocompromised patient, she was at greater risk for infection and required close observation, careful aseptic practices, and an understanding of the differences in symptoms associated with the immunocompromised state.
According to the CDC, when a vascular access device is required, clinicians should choose the least invasive device with the lowest risk of complications.(3) Additionally, the 2011 Infusion Nurses Society (INS) Standards of Practice recommends selecting the smallest gauge and length of a catheter with the fewest number of lumens to accommodate and manage prescribed therapy.(12) At the time of PICC insertion, the vein-to-catheter ratio should be identified to reduce the risk of thrombosis. Catheter–vein ratio is measured at the time of ultrasound assessment and prior to the insertion of the catheter. Evaluation of the vein is done with ultrasound visualization and measurement. When the catheter size exceeds 50% of the diameter of the vein, the risk of venous thrombosis increases dramatically. The risk of thrombosis is also higher in patients with obesity, cancer, diabetes, and catheter insertions with two or more attempts.(6) Greater emphasis is now being placed on risk assessment through measurement of the vein's diameter in a natural state, without a tourniquet, to determine catheter–vein ratio prior to catheter insertion.(13) Though facilities prefer larger dual and triple lumen PICCs, affording clinicians more access for medication and fluid administration, these additional lumens increase the risk of infection and larger diameter lumens increase the risk of thrombosis.(14) When patients receive intravenous medications in the home, the use of a single lumen catheter is adequate and serves as a way to reduce risk of both thrombosis and infection.
Technology also plays a part in establishing safety for a patient. There are now antimicrobial and antithrombotic catheters available that reduce risk for patients. The use of antimicrobial catheters is supported by randomized trials and economic evaluations establishing their efficacy.(15,16) A 2008 economic analysis from the National Health Trust of the United Kingdom concluded that anti-infective CVCs should be integrated into standard care because they are clinically effective, relatively inexpensive, and offer potential cost savings, but that "the use of these anti-infective catheters without the appropriate use of other practical care initiatives will have only a limited effect on the prevention of CRBSIs."(15) Additional items that reduce the risk of contamination or infection of the catheter include prefilled syringes, disinfecting caps for access points, antimicrobial sponges or dressings for the insertion site, and catheter securement devices. Each technology reduces complications, aids those who manage the PICC, and results in better outcomes.
Conclusion
The case presented represents the diverse and sometimes fractured approach to the delivery of intravenous treatments. Coordination of care is challenging, especially when treatment is continued into the home. It requires the establishment of a plan and education for providers and patients to safely reach the goals of treatment. PICCs have performed well in home care and outpatient environments for more than 20 years with demonstrated low infection rates.(8) Education is frequently undervalued and underutilized in our busy practices, but the results are clear. Each time education is provided, infection and other complications are reduced.(17)
Take-Home Points
- PICC placement and utilization continue to grow in hospital settings; specialized PICC teams and services can reduce the incidence of complications and achieve desired safety outcomes.
- Infection and thrombosis are the two most common complications.
- Along with education and training, adoption of a central line bundle of safety practices is recommended to reduce the risk of infection associated with PICC placement.
- Establish a plan for PICC management with patients that require management outside the insertion facility (e.g., visiting nurse, validation that clinicians are trained to manage PICCs, education for the patient).
- Apply guidelines and strategies for infection prevention with insertion and management practices with PICCs.(18)
Nancy L. Moureau, BSN, RN, CRNI, CPUI, VA-BC CEO PICC Excellence, Inc.
References
1. iData Research. US Market for Vascular Access Devices and Accessories 2008. [Available at]
2. iData Research. US Market for Vascular Access Devices and Accessories 2010. [Available at]
3. O'Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections, 2011. [Available at]
4. Mehall JR, Saltzman DA, Jackson RJ, Smith SD. Fibrin sheath enhances central venous catheter infection. Crit Care Med. 2002;30:908-912. [go to PubMed]
5. Raad II, Luna M, Khalil SM, Costerton JW, Lam C, Bodey GP. The relationship between the thrombotic and infectious complications of central venous catheters. JAMA. 1994;271:1014-1016. [go to PubMed]
6. Timsit JF, Farkas JC, Boyer JM, et al. Central vein catheter–related thrombosis in intensive care patients: incidence, risks factors, and relationship with catheter-related sepsis. Chest. 1998;114:207-213. [go to PubMed]
7. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159-1171. [go to PubMed]
8. Moureau N, Poole S, Murdock MA, Gray SM, Semba CP. Central venous catheters in home infusion care: outcomes analysis in 50,470 patients. J Vasc Interv Radiol. 2002;13:1009-1016. [go to PubMed]
9. Royer T. Implementing a better bundle to achieve and sustain a zero central line–associated bloodstream infection rate. J Infus Nurs. 2010;33:398-406. [go to PubMed]
10. Pronovost PJ, Berenholtz SM, Goeschel C, et al. Improving patient safety in intensive care units in Michigan. J Crit Care. 2008;23:207-221. [go to PubMed]
11. AHRQ Patient Safety Project Reduces Bloodstream Infections by 40 Percent. Rockville, MD: Agency for Healthcare Research and Quality; September 10, 2012. [Available at]
12. Infusion Nurses Society. 2011 Infusion Nursing Standards of Practice. J Infus Nurs. 2011;34(suppl 1s):S1-S110.
13. Nifong TP, McDevitt TJ. The effect of catheter to vein ratio on blood flow rates in a simulated model of peripherally inserted central venous catheters. Chest. 2011;140:48-53. [Available at]
14. Trerotola SO, Stavropoulos SW, Mondschein JI, et al. Triple-lumen peripherally inserted central catheter in patients in the critical care unit: prospective evaluation. Radiology. 2010;256:312-320. [go to PubMed]
15. Hockenhull JC, Dwan K, Boland A, et al. The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation. Health Technol Assess. 2008;12:1-154. [go to PubMed]
16. Crnich CJ, Maki DG. Are antimicrobial-impregnated catheters effective? Don't throw out the baby with the bathwater. Clin Infect Dis. 2004;38:1287-1292. [go to PubMed]
17. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30:59-64. [go to PubMed]
18. Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line–associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S22-S30. [go to PubMed]
Figure
Figure. Example of a double-lumen peripherally inserted central catheter (PICC) placed in the arm of a patient.