Getting a Good Report Card: Unintended Consequences of the Public Reporting of Hospital Quality
Lindenauer PK. Getting a Good Report Card: Unintended Consequences of the Public Reporting of Hospital Quality. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2006.
Lindenauer PK. Getting a Good Report Card: Unintended Consequences of the Public Reporting of Hospital Quality. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2006.
Case Objectives
- Understand the rationale for public reporting of hospital quality.
- List the current measures set being used nationally.
- Describe unintended consequence of public reporting and interventions to prevent these.
The Case
A 55-year-old woman with end stage renal disease requiring hemodialysis and coronary artery disease (status post-coronary artery bypass grafting and placement of a St. Jude prosthetic valve) was admitted to the medical service with palpitations and chest pain. She reported missing her scheduled hemodialysis session, and her symptoms resolved with prompt inpatient hemodialysis. In the course of her work-up, it was noted that she was subtherapeutic on her anticoagulation with warfarin, and a heparin drip was initiated with the plan to bridge her until her INR was in the therapeutic range.
Mostly in response to increasing pressure from the hospital's administration to improve compliance with publicly reported quality measures, the attending physician recommended pneumococcal vaccination, and it was administered. Later that day, the patient complained of pain over her right upper arm. The attending told the patient that this was a common complaint after immunization and that it would resolve. The next day, the patient reported that her pain was worse, and the team noted an 8-centimeter hematoma within the muscles of her upper arm. The hematoma resolved spontaneously. There was no permanent harm.
The Commentary
Since first proposed by pioneers like Ernest Codman and Florence Nightingale, the public reporting of measures of hospital quality has been viewed with skepticism and at times outright scorn by members of the medical profession. Despite these protests, there has been an explosion of public reporting over the last few years, in America and around the world.
The public release of hospital quality measures is typically undertaken with a number of explicit and implicit aims in mind.(1) Ideally, public reporting improves transparency and can empower patients to make better choices about where to seek treatment. This increases hospital accountability for quality of care and provides a stimulus to improve for fear of losing market share to local competitors. Public reporting also enables the stewards of the public's health (including government, regulators, accreditors, and payers) to track performance over time, and these data can be tied to compensation through "pay-for-performance" contracts.
Public reporting begins with the selection of evidence-based processes or outcomes to be measured and the identification of meaningful opportunities to improve practice. There are many organizations currently involved in the design and implementation of public reporting in the United States. The most important of these is the Hospital Quality Alliance (HQA), a national public-private collaboration established to encourage hospitals to voluntarily collect and report hospital quality performance information.(2) All acute care hospitals in the United States have been invited to participate in this initiative, and by linking participation in the program to the annual Medicare payment update, the Centers for Medicare and Medicaid Services has achieved participation rates of greater than 98%. The HQA began in late 2003 with a limited set of 10 quality measures spread across three clinical conditions: heart failure, acute myocardial infarction, and pneumonia. By 2006, the program had expanded to include 21 measures (Table) related to the original three clinical areas, with the addition of new measures focused on the quality of surgical care.
Despite the natural appeal of public reporting, relatively little is known about its actual impact on quality of care or patient outcomes. After New York State began publicly reporting hospital-specific mortality following coronary bypass surgery, mortality rates were observed to fall, but the mechanism through which this occurred continues to be debated.(3,4) Similarly, a report on the impact of the "Quality Counts" program in Wisconsin found that hospitals involved in public reporting of risk-adjusted outcomes engaged in more quality improvement activities and were more likely to show improved outcomes than control institutions.(5) Although these limited data support one of the premises of transparency—that they will catalyze hospitals' quality improvement activities—the jury is still out about the impact of public reporting on patient decisions regarding where to obtain medical care. What little is known suggests that hospital market share has been largely unaffected by these initiatives.(6)
Concerns over Public Reporting
Concerns expressed about public reporting can be grouped into two major categories—those that question the validity of the undertaking itself and those that raise questions about its unintended consequences.(7-11) Among the first group, it is often pointed out that the field of quality measurement in health care is still in its infancy and there are as of yet only a small number of measures based on sound evidence. Further, measures that have been validated for one purpose are sometimes used inappropriately for other purposes. For example, patient safety indicators derived from administrative data sources can be valuable tools for case identification and the monitoring of rates at a single organization but should not be used to compare rates across hospitals. For example, Romano and colleagues reported that, when derived from administrative data sources, more than half of the observed variation in risk-adjusted rates of postoperative infections seen across hospitals could be attributed to differences in coding practices and not actual outcomes.(12) Nonetheless, some health plans have begun to grant subscribers access to data that compare hospitals in a region.
Additionally, attempts to compare outcomes across institutions are hampered by both real and perceived challenges of carrying out risk adjustment and by limited statistical power when studying rare events. While this has led to a gradual shift toward the use of process measures (what was done) instead of outcomes (what happened to the patient), improving the performance of process measures may fail to result in actual improvements in the outcomes of care. This can occur when the association between the process measure and the desired outcome is weak, or when there is improvement in the documentation of the process but not in the actual process itself. This latter example may lead hospitals to believe that they have improved care when in fact they have simply improved documentation.
The second concern expressed about the public reporting of quality data relates to the phenomenon of unintended consequences. One framework for classifying unintended consequences distinguishes those that result in direct harm from those that cause harm indirectly. There are several types of events that can lead to direct harm. The development of an allergic reaction following medication administration in a patient not previously known to have an allergy is one example of direct harm. On the other hand, direct harm is more predictable when errors are made or guidelines are violated. The administration of a beta blocker to a patient with heart block and the provision of multiple influenza vaccinations to a patient during a single hospitalization are both examples of this type of unintended consequence. Finally, direct harm can occur when caregivers are faced with diagnostic or therapeutic uncertainty, and this risk can be exacerbated by the incentives created by public reporting. For example, an initiative designed to increase the use of venous thromboembolism prophylaxis that did not adequately specify contraindications to the use of heparins is likely to produce excessive rates of bleeding. In another example, the current standard that antibiotics should be given to patients with pneumonia within 4 hours of arrival in the ED may result in antibiotics being given to some patients who prove to have heart failure or pulmonary embolism.(11)
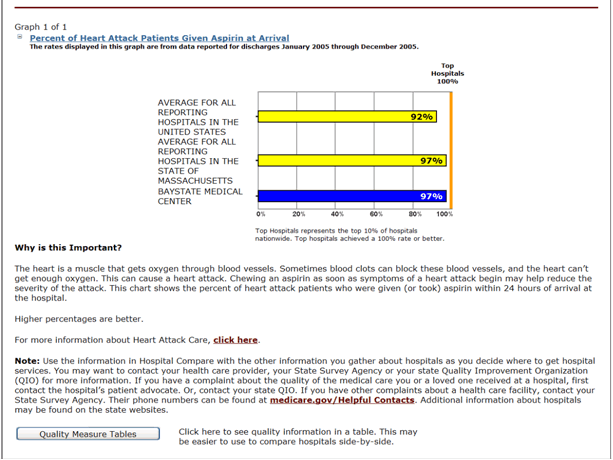
While less apparent, the problem of indirect harm may be just as great as or greater than the examples described above. First, patients may have important health care matters neglected when physicians or nurses shift their attention to those aspects of care for which they are being asked to report publicly. Consider the pyrrhic victory of achieving optimal glycemic control during the hospitalization of a diabetic patient while failing to intervene to correct untreated hyperlipidemia. Furthermore, hospitals that "play to the test" by reallocating valuable human and other resources to excel in public measures may overlook more pressing opportunities to improve the care of patients with conditions not subject to public reporting. For example, hospitals may decide to hire additional clinical personnel to improve performance from 98% to 100% on a measure such as the use of aspirin in myocardial infarction while the quality of care for patients with stroke or COPD is neglected. Another concern that has been raised about public reporting, especially when outcomes such as mortality or complications are being compared, is the risk that hospitals will turn away high-risk patients who might tarnish their scores. Finally, patients and payers may misinterpret quality performance information and make poor choices about where to seek care or direct patients. This is a significant risk given the limited number of clinical areas for which public reporting has been implemented and in light of the fact that strong performance in one area does not predict such performance in other clinical areas.(13)
Reducing the Burden of Unintended Consequences
Given the many ways in which quality improvement and public reporting can result in unintended consequences, no single approach can be relied upon to prevent all problems. First and foremost, hospitals must recognize that unintended consequences are bound to occur following any effort to implement change. Failure Mode Effects Analysis (FMEA) is a systematic, proactive method for evaluating a process to identify where and how it might fail, and to assess the relative impact of different failures. In other words, FMEA specifically concerns itself with attempting to anticipate unintended consequences and should be incorporated into the planning for any major quality improvement project.
Education is the foundation of good practice and remains a necessary though not sufficient way to prevent errors. Education must be supplemented with checklists, protocols, and other reminder systems, and whenever possible, computers can and should be employed to minimize the need to rely on human vigilance. For example, electronic order entry systems can and are being used to prevent the administration of medications to patients with specific contraindications. Forcing functions are another approach that can be used to reduce the risk of unintended consequences. For example, a medication-dispensing and administration system can be designed to require the nurse to document a heart rate before releasing a beta blocker for administration. Monitoring is an important and probably underutilized approach to addressing the problem of unintended consequences; underutilized because unintended consequences are not always incorporated into the quality improvement planning process and because quality improvement staffs are stretched thin complying with current reporting requirements. However, the automated detection of adverse events was described more than 15 years ago and can dramatically reduce the burden of data collection.(14) For example, automated detection can be used to measure how often patients with heart failure receive treatment with antibiotics intended for treatment of pneumonia, or how frequently patient on narcotics are administered opiate antagonists like naloxone to reverse oversedation or respiratory depression.
Payers also have an important role to play in ensuring that unintended consequences do not diminish the benefits gained through public reporting. First, they must set and communicate realistic goals. For many measures, a goal of 100% performance is not possible without resulting in an unacceptable rate of unintended effects. For example, it is unlikely that hospitals can deliver antibiotics to 100% of patients with pneumonia within 4 hours without exposing many other patients to antibiotics unnecessarily. By instituting a broad range of measures, by rotating them frequently, and by not announcing beforehand which will be made public, payers may also be able to prevent hospitals from gaming the system. Finally, where appropriate, payers should consider incorporating predictable unintended events into the standard public reporting measure sets.
The Case Revisited
How can this particular case be viewed in light of the previously outlined framework? First, the current immunization measures apply only to patients with pneumonia, so while this patient is likely to have benefited from immunization, there was in fact no such reporting requirement, and the attending physician was not correct in his or her belief that failure to immunize would have affected the hospital's performance ratings. Second, because anticoagulation is not considered a contraindication to the administration of immunization (nor to intramuscular injection more generally), this is probably best viewed as a nonpreventable case of direct harm that occurred while following clinical guidelines. Although hematoma formation and frank bleeding are recognized risks following both subcutaneous and intramuscular injections, the risk does not appear to be significantly increased among patients on anticoagulants, and thus the benefits of immunization outweighed the risk of complication.(15-18)
Certainly, had the patient's partial thromboplastin time (PTT) been extremely elevated, it would have been prudent to wait until it had fallen back to a therapeutic range before immunizing. More importantly, if there was reasonable doubt on the part of the team responsible for this patient's care that administration of the immunization was either relatively or absolutely contraindicated, then professionalism dictates that they set aside the hospital's interests in favor of the patient's. This is especially important to appreciate given that specifications for existing quality measures do not enumerate every possible scenario that might make immunization contraindicated, as this is neither possible nor practical from the standpoint of chart review. This is another example of why performance rates of 100% are not always possible or desirable. Finally, regulatory agencies must be willing to modify problematic measures. For example, if a sufficient number of hospitals reported that patients on heparins experienced hematomas following immunization, then the measure ought to be revised to reflect this.
Peter Lindenauer, MD, MSc Medical Director, Clinical and Quality Informatics; Baystate Health Assistant Professor of Medicine; Tufts University School of Medicine
Faculty Disclosure: Dr. Lindenauer has declared that neither he, nor any immediate member of his family, has a financial arrangement or other relationship with the manufacturers of any commercial products discussed in this continuing medical education activity. In addition, the commentary does not include information regarding investigational or off-label use of pharmaceutical products or medical devices.
References
1. Marshall MN, Shekelle PG, Leatherman S, Brook RH. The public release of performance data: what do we expect to gain? A review of the evidence. JAMA. 2000;283:1866-1874. [go to PubMed]
2. Hospital Quality Alliance Web site. Available at: http://www.aha.org/aha/key_issues/qualityalliance/index.html. Accessed November 1, 2006.
3. Omoigui NA, Miller DP, Brown KJ, et al. Outmigration for coronary bypass surgery in an era of public dissemination of clinical outcomes. Circulation. 1996;93:27-33. [go to PubMed]
4. Burack JH, Impellizzeri P, Homel P, Cunningham JN Jr. Public reporting of surgical mortality: a survey of New York State cardiothoracic surgeons. Ann Thorac Surg. 1999;68:1195-1200; discussion 1201-1102. [go to PubMed]
5. Hibbard JH, Stockard J, Tusler M. Hospital performance reports: impact on quality, market share, and reputation. Health Aff (Millwood). 2005;24:1150-1160. [go to PubMed]
6. Jha AK, Epstein AM. The predictive accuracy of the New York State coronary artery bypass surgery report-card system. Health Aff (Millwood). 2006;25:844-855. [go to PubMed]
7. Eddy DM. Performance measurement: problems and solutions. Health Aff (Millwood). 1998;17:7-25. [go to PubMed]
8. Epstein AM. Rolling down the runway: the challenges ahead for quality report cards. JAMA. 1998;279:1691-1696. [go to PubMed]
9. Casalino LP. The unintended consequences of measuring quality on the quality of medical care. N Engl J Med. 1999;341:1147-1150. [go to PubMed]
10. Werner RM, Asch DA. The unintended consequences of publicly reporting quality information. JAMA. 2005;293:1239-1244. [go to PubMed]
11. Wachter RM. Expected and unanticipated consequences of the quality and information technology revolutions. JAMA. 2006;295:2780-2783. [go to PubMed]
12. Romano PS, Chan BK, Schembri ME, Rainwater JA. Can administrative data be used to compare postoperative complication rates across hospitals? Med Care. 2002;40:856-867. [go to PubMed]
13. Jha AK, Li Z, Orav EJ, Epstein AM. Care in U.S. hospitals—the Hospital Quality Alliance program. N Engl J Med. 2005;353:265-274. [go to PubMed]
14. Classen DC, Pestotnik SL, Evans RS, Burke JP. Computerized surveillance of adverse drug events in hospital patients. JAMA. 1991;266:2847-2851. Erratum in: JAMA. 1992;267:1922. [go to PubMed]
15. Pneumovax 23 [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 1986.
16. Raj G, Kumar R, McKinney WP. Safety of intramuscular influenza immunization among patients receiving long-term warfarin anticoagulation therapy. Arch Intern Med. 1995;155:1529-1531. [go to PubMed]
17. Delafuente JC, Davis JA, Meuleman JR, Jones RA. Influenza vaccination and warfarin anticoagulation: a comparison of subcutaneous and intramuscular routes of administration in elderly men. Pharmacotherapy. 1998;18:631-636. [go to PubMed]
18. Treadwell T. Intramuscular injection site injuries masquerading as pressure ulcers. Wounds. 2003;15:302-312.
Table
Hospital Quality Alliance: Current Measure Set
| Heart Attack (Acute Myocardial Infarction or AMI)
Heart Failure
Pneumonia
Surgical Infection Prevention
|
Figure
Example Screenshot of HQA Data Available to the Public on the Hospital Compare Web Site [http://www.hospitalcompare.hhs.gov/].