An Inadvertent Bolus of Norepinephrine.
Fazio S, Blackmon E, Doroy A, et al. An Inadvertent Bolus of Norepinephrine.. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2021.
Fazio S, Blackmon E, Doroy A, et al. An Inadvertent Bolus of Norepinephrine.. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2021.
Patrick Romano, MD, MPH; Debra Bakerjian, PhD, APRN, RN; Sarina Fazio, PhD, RN; Emma Blackmon, PhD, RN, CCRM; Amy L. Doroy, PhD, RN, NEA-BC; Ai Nhat Vu; Paul MacDowell, PharmD, BCPS; Ulfat Shaikh, MD; Patricia Poole, PharmD for this Spotlight Case and Commentary have disclosed no relevant financial relationships with commercial interests related to this CME activity.
Learning Objectives
- Explain general approaches for treating hypotension in the ICU
- Recognize risks associated with vasopressor (norepinephrine) administration
- Identify the most frequent types of IV medication errors in the ICU
- Describe best practices for co-administration of multiple IV infusions
The Case
A 64-year-old woman with a history of anxiety, depression, hypothyroidism, arthritis, paroxysmal atrial fibrillation, an ascending aortic aneurysm, and a bicuspid aortic valve, presented to clinic with several months of worsening dyspnea on exertion. An echocardiogram showed moderate-to-severe aortic stenosis. She then underwent surgery for an aortic valve replacement, ligation of her left atrial appendage, and repair of her ascending aortic aneurysm.
Following surgery, the patient experienced intermittent episodes of hypotension, for which she was given intravenous (IV) fluid boluses and vasopressor support. She received IV norepinephrine at a rate of 0.5 - 6 mcg/minute until 21:00 on postoperative day 1. At 08:00 on postoperative day 2, the patient’s blood pressure was 98/59 mmHg and a 250 mL fluid bolus was ordered. The fluid bag was attached to the IV line that had the vasopressor at a Y-site and the bolus was initiated. The patient developed diaphoresis, tachycardia to 114 bpm, and hypertension with an apex value of 271/161 mmHg. Once the inadvertent bolus was recognized, the vasopressor infusion was immediately stopped. In total, the patient received approximately 4.5 mL (or 160 micrograms) of norepinephrine infused over 15 minutes.
The patient was then closely monitored, and her hemodynamic parameters returned to baseline approximately 15 minutes later. However, the patient had ongoing hypotension in the hours following the inadvertent bolus of norepinephrine with a nadir of 54/38 mmHg, again requiring vasopressor administration and additional fluid boluses. The next day, the patient's blood pressure stabilized, and she was transferred to a stepdown unit, and later discharged home.
While the incident caused only temporary and minor harm to the patient, it was a cause of significant stress and anxiety throughout the rest of her hospital stay and persisted after her discharge. Under different circumstances, this error could have resulted in significant harm, including neurologic impairment and death.
The Commentary
By Sarina Fazio, PhD, RN, Emma Blackmon, PhD, RN, Amy Doroy, PhD, RN, Ai Nhat Vu & Paul MacDowell, PharmD
ICU Hypotension
Hypotension following cardiac surgery may result from a variety of factors, such as hypovolemia, pump failure due to heart failure or shock, or maldistribution of blood flow due to septic shock.1-3 Severe, systemic vasodilation can occur in 5-25 % of patients following cardiac surgery, resulting in postoperative hypotension despite a normal or increased cardiac index.2,4 Most patients with vasodilatory shock respond to hemodynamic-guided IV fluid therapy and/or low-dose vasopressor agents, such as norepinephrine or vasopressin.3,5,6
Expected mean arterial pressure (MAP) values in the postoperative period are between 60-90 mmHg. Vasopressors are indicated for a MAP < 60 mmHg, a decrease in systolic blood pressure > 30 mmHg from baseline, or when there is risk of end-organ dysfunction due to hypotension.7,8 Prior to initiation of vasopressor therapy, patients should be assessed for hypovolemia which should be corrected with intravascular volume resuscitation,9 as vasopressors may be only partially effective in the setting of coexistent hypovolemia.10 However, for patients with pulmonary edema due to heart failure or acute respiratory distress syndrome, fluids may be cautiously withheld and/or administered in smaller quantities to assess for fluid responsiveness and prevent fluid overload.11,12
Vasopressor Administration & Monitoring
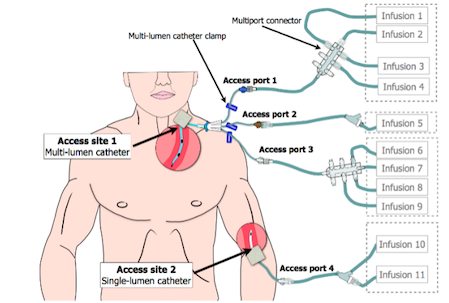
Vasopressors are drugs that induce vasoconstriction and elevate MAP.7 They are most safely administered intravenously through a central venous catheter to prevent risk of peripheral extravasation and to facilitate rapid, systemic distribution.13 Despite their life-sustaining benefit, vasopressors and inotropic agents have the potential, at high doses and with prolonged use, to cause serious complications, such as cardiac arrhythmias, myocardial ischemia, peripheral vascular insufficiency and peripheral ischemia.14 Vasopressor administration requires admission to an ICU and continuous cardiac and blood pressure monitoring by an interprofessional team. Infusions are typically titrated by ICU nurses based on provider orders regarding clinical endpoints and hemodynamic goals, such as blood pressure (MAP) and end-organ perfusion, that may differ based on clinical condition.15,16 Figure 1, adapted from Pinkney and colleagues,17 depicts a multi-lumen central venous catheter connected to multiple IV infusions.
Figure 1. Multiple IV Infusion Setup
Image adapted and printed with permission from Pinkney et al., 201417
Norepinephrine is the preferred, first-line vasopressor for both the treatment of septic and distributive shock.18 Norepinephrine produces vasoconstriction and increases contractility by stimulating alpha and beta1 adrenergic receptors.7 Norepinephrine has a rapid onset of action, with 5 minutes to peak serum steady-state and mean half-life elimination of 2.4 minutes. Norepinephrine can be administered using weight-based or non-weight-based dosing, the latter of which is calculated and rounded for an 80 kg patient. Though dosing and titration parameters may vary across institutions and clinical pathologies, examples of initial dosing and dose ranges are provided in Table 1.15,16
| Table 1: Norepinephrine Dosing | ||
|---|---|---|
| Initial Dose | Typical Dosage Range | |
| Weight-based Dosing | 0.05-0.15 mcg/kg/minute | 0.05 to 0.4 mcg/kg/minute |
| Non-weight-based Dosing | 5-15 mcg/minute | 5 to 30 mcg/minute |
ICU Medication Errors Associated with IV Infusions
IV medication administration is an integral component of treating ICU patients. The complexity of medication administration, which can require up to 200 steps from prescription to administration,19 combined with high patient acuity and treatment complexity, makes ICU medication administration particularly error-prone.20 Additional factors such as a high number of infusions, administration of high-alert medications, and rapid bolus infusions further increase the likelihood of an IV medication error taking place and a subsequent adverse drug event.21 The Institute for Safe Medication Practices (ISMP) has reported that “56% of medication errors are associated with IV medications,”22 and medication administration is reported to be the most common step at which errors occur, accounting for approximately 66% of ICU medication errors.23
There is substantial heterogeneity in the most prevalent types of IV medication errors in ICU settings, due to differences in data collection methodologies and practices across hospitals. For instance, a prospective, observational Canadian ICU study pre-dating EHR implementation found that incomplete documentation accounted for 92.7% of the IV medication errors observed, whereas inappropriate Y-site or piggyback infusion errors accounted for 6.7% of IV administration errors.20 In contrast, Fahimi and colleagues23 observed that a majority of IV medication errors were related to bolus administration (43.4%) and incorrect infusion rate (23.0%).24 An international study published in 2011 also found errors in bolus IV medication administration to be common and associated with significantly higher error rates than continuous infusions (77.2% vs 47.7%; p<0.0001).25 While overall error rates vary widely, comparable rates (determined using similar measurement methods) of potentially harmful errors (those rated D or above, indicating the majority of patients require extra monitoring or intervention to prevent harm) lie between 0.4-3.8%.26-29
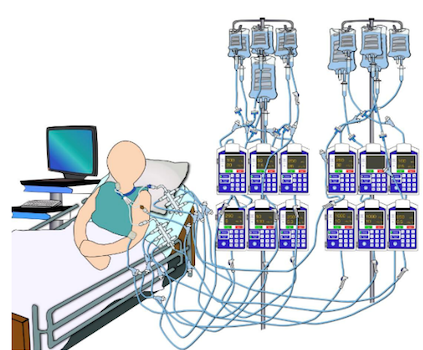
Rate or IV Line Mix-ups
In 2015, the Emergency Care Research Institute (ECRI) rated infusion mix-ups as one of the top 10 health technology hazards.30 In the ICU setting, where patients commonly receive multiple IV infusions, IV line or rate mix-ups are common errors that can be attributed to a number of factors including a variety of medication administration routes, difficulties in visually distinguishing between lines (see Figure 2), and inadequate medication reconciliation handoffs.17,30 In a report by the Pennsylvania Patient Safety Advisory, rate and line mix-ups accounted for 23% (n=205) of reported events,24 and most commonly occur with high-alert medications (92%). While there are many types of IV infusion mix-up errors, these types of errors commonly result in medication dosing errors and incorrect amounts of fluid volume delivered to the patient.17
Figure 2. Multiple IV Infusion Administration
Image adapted and printed with permission from Pinkney et al., 201417
Errors Associated with Secondary Infusions
Secondary (“piggyback”) infusions are IV infusions that are attached to the primary continuous IV infusion and hung above the primary infusion bag to deliver intermittent, scheduled medications, such as antibiotics and electrolytes. Medication errors associated with secondary infusions arise from their complex setup process and include but are not limited to wrong Y-site connection, failure to open the catheter clamp, inappropriate height differential between the primary and secondary infusion bags, and programming errors.24 Secondary infusion errors accounted for approximately 39% of multiple IV infusion incidents reported to the U.S. Food and Administration (FDA) in 2008, 45% of which resulted in patient harm.31
Errors Associated with Norepinephrine
The ISMP includes adrenergic agonists (e.g., norepinephrine) among medications that have a heightened risk of causing significant patient harm if used in error.32 Although vasoactive drugs are a class of therapeutic drugs among those most commonly associated with medication errors in adult ICUs and are administered in ICUs globally,33 errors related to vasopressor administration through continuous infusions are infrequently discussed in the literature. The earliest report the authors of this commentary could find of an inadvertent over-infusion of norepinephrine related to a pump programming error that resulted in cardiac arrest and the patient’s subsequent death was published in 2015.34 Given the complexities of dosing and titration when administering norepinephrine, additional research is necessary to identify and examine the effects of risk reduction strategies on patient outcomes.
Best Practices in Management of Multiple IV Infusions
Over the past 10 years, the potential risks associated with administration of multiple IV infusions in the ICU setting have been increasingly recognized. In 2010, the Association for the Advancement of Medical Instrumentation and the U.S. FDA issued a call to action to improve the management of multiple IV infusions.31,52 Here, we briefly outline some recent leading efforts and system-level approaches to improving vasopressor and multiple IV infusion safety.
Utilization of Smart Pumps
The use of smart pumps with ‘dose error reduction software’ is becoming more prevalent as a method to reduce the risk of errors associated with IV infusions and pump programming.35 In addition to comprising a drug library, smart pump software of this type is programmed with individual medication parameters, such as acceptable prescribed rate, concentration, and dosing limits.34 While use of smart pumps has the potential to mitigate errors associated with incorrect programming of IV infusions, current smart pumps do not target risks associated with setting up and co-administration of multiple IV infusions.17,24,27 In this regard however, the medication error that occurred in the case that is the focus of this commentary was related to the physical maintenance of the patient’s IV infusions, an underappreciated area of concern that lacks a technological, smart pump prevention solution.
Standardized Dosing
Though norepinephrine is always administered through an infusion pump, there are additional institutional steps that can be taken for norepinephrine administration that may increase safety. We previously discussed (see section on Vasopressor Administration & Monitoring above) how norepinephrine can be administered using either a weight (mcg/kg/min) or non-weight-based (mcg/min) strategy. Variations in how ICU nurses manage vasoactive medications and identification of practice variations which contribute to medication errors, patient harm and nurse anxiety were the focus of a recent systematic review.36 As reported in the review, ICU nurses aligned their choices of dosing units with the preferences of the patients’ primary medical team; surgical teams are known to favor weight‐based doses while medical teams prefer non‐weight‐based units.37
Conversion to a single method of dosing (e.g., weight-based dosing) can be implemented to prevent errors associated with medication administration. In addition, standardizing vasopressor infusion concentration across care areas and provider services may also prevent norepinephrine IV errors. Standard concentrations of norepinephrine infusions are either 16 mcg/mL (4 mg in 250 mL) or 32 mcg/mL (8 mg in 250 mL) of 5% dextrose in water or normal saline.38 However, if an order to change norepinephrine concentration occurs during medication administration, implementing effective communication or an alerting system is an important additional step to take to ensure smart pump programming updates are reflected and medication/tubing is exchanged properly. For all of these reasons, implementing a standardized dosing strategy has the potential to reduce the risk of medication errors by standardizing preparation and programming of vasoactive infusions.36,37,39-41
IV Infusion Setup
Setting up multiple, continuous IV infusions is a common nursing task when caring for a critically ill patient in the ICU. Each additional IV infusion can increase the likelihood of an error occurring by 3% due to increasing demands associated with a) physically managing multiple infusions with limited access points and b) cognitively managing multiple medication orders and titration parameters.21 Research has shown that setting up multiple infusions in parallel, when either initiating IV therapy or changing medications and their tubing, has led to errors related to IV tubing, pumps, drug orders and mixed up labels.42-44 Instead, to decrease the chance of error when setting up multiple IV infusions, it is recommended that each IV infusion be set up one at a time and as completely as possible before moving on to the next infusion.42-45 Furthermore, clinicians should “trace” infusions from top to bottom, or from the administration bag, through the pump, and to the patient, before making any new connections or disconnections, when adjusting any existing medication rates, and during communication handoffs.
Line Labeling
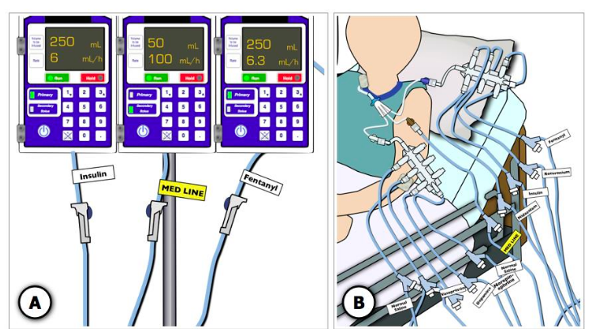
Several technological solutions have been proposed to improve accurate identification of IV infusions when multiple infusions are being administered. These solutions include color-coded tubing, pre-printed or handwritten adhesive labels, infusion organizers, pump displays, and light-linking systems.17 While additional research will be required to determine which of these solutions results in the fewest errors, the findings from a high-fidelity critical care simulation study suggest that line labels/organizers increase infusion identification accuracy and efficiency.46 However, this solution would require standardization in labelling practices to decrease troublesome variation. For example, ISMP supports 1) use of black and white medication labels (with the exception of the emergency medication or “stat” line label) to promote careful reading to differentiate between infusions47,48 and 2) placement of labels in two standard locations, below the smart pump and near the distal end of the tubing49,50 (see Figure 3). Lastly, line labeling should not be the sole means used to identify medication infusions; the labels should support clinicians in facilitating line tracing.24
Figure 3. Labeling Multiple IV Infusions
Image adapted and printed with permission from Pinkney et al., 201417
Management of Continuous Primary Infusions
- Dedicated Lines: High-alert, continuous medications should be administered as a primary infusion (on its own dedicated line) without any attachment of a secondary/piggyback infusion.42 Attachment of a secondary infusion may result in transition of a continuous medication to an intermittent one and delivery of inconsistent doses and rates outside of what is intended for medication delivery.24 Vasopressors such as norepinephrine should be administered through a dedicated IV line or administered concurrently with other compatible medications though a Y-site multi-port connector when vascular access is limited.
- Extension Sets: To administer continuous medications through a dedicated IV line, Y-site or multi-port connectors/IV extension sets are used to simultaneously deliver IV medications. Back-check valves, which prevent backflow of medication due to differences in pressure in an IV infusion line, should also be used to prevent errors associated with administering multiple IV infusions, at different rates and quantities, into the same access point.42 There is substantial heterogeneity in types of IV tubing, extension sets and multi-port connectors and, depending on the institution and what is in stock when ordering, what is available for use can vary. There are no standard guidelines for setting up multiple primary continuous IV infusions and multi-port connectors, especially for life-sustaining therapies that cannot be interrupted without causing hemodynamic instability.42 As a result, processes and practices differ from nurse to nurse and within and across departments.17 Standardization of IV tubing and extension equipment across an institution may help to validate and implement best practices. Further research is necessary to understand practice variation in setting up, flushing, and exchanging multi-port connectors.
- IV Bolus: Bolus administration refers to rapid infusion of a pre-determined volume or dose of medication or fluid to the patient, either through an infusion pump, via gravity/pressure bag or pushed manually through a syringe into an IV line. Bolus infusions should be administered through a dedicated emergency medication (“stat”) IV line and a single vascular access port to avoid Y-site incompatibility and inadvertent rapid infusion of other medications. Ideally, bolus infusions should not be co-administered with additional medications or attached on a primary IV tubing side port. However, in situations with limited vascular access, co-administration may occur during the process of establishing IV access for medication and fluid administration. In cases where IV push medication is prescribed, the ISMP suggests administering IV push medications through a dedicated IV infusion line, through the port closest to the patient, unless contraindicated or inaccessible for use, such as during a sterile procedure.51
Titratable, Intermittent, Low-Dose Infusions:
During titration for reduction of vasopressors such as norepinephrine, patients can have periods of time when they are receiving very low and intermittent doses, until their MAP is consistently > 60 mmHg. In these cases, the nurse may decide to keep the IV infusion attached to the central venous catheter to reduce the risk of central-line-associated blood stream infection (CLABSI). In contrast, for patients with labile blood pressure, when vasopressors are being titrated on and off according to medication titration orders, the nurse may disconnect the IV tubing from the patient's central venous catheter port but leave the bag/tubing present and hanging in the room in case the patient requires restarting the vasopressor due to a subsequent drop in blood pressure. However, once the medication order is discontinued, removing intravenous bags and tubing from the patient comprises current best practices. Lastly, vasopressor titration is another high-profile patient safety topic; however, discussion of the intricacies of vasopressor titration is outside the scope of this commentary.
Important Considerations
Several recommendations for co-administration of multiple IV infusions are outlined in this commentary. However, there are many additional considerations clinicians must account for in “real-world” settings given the nature and complexity of caring for critically ill patients in the ICU setting. Some of the considerations that have not been examined herein include:
- Additional risk of infection (e.g., CLABSI) when connecting and disconnecting IV infusions from central venous catheter access ports,
- Limited availability of vascular access coupled with co-administration of many infusions (e.g., >10) with different compatibilities,
- The fact that technology does not solve all the problems with IV administration, human factors must be considered as well, and
- The COVID-19 pandemic, which has led to changes in: the IV supply chain, nurse-to-patient ratios, and handoff communication practices and independent medication checks to decrease virus exposure and PPE waste.
Conclusion
While administration of multiple IV infusions is ubiquitous in the ICU, and there are several established safety parameters associated with medication concentration, dosing and pump programming, the physical dexterity required for administering multiple IV infusions concurrently is underappreciated and lack thereof can, in fact, lead to serious harm. More research into this patient safety issue should be conducted. Also, standard guidelines detailing safe practices for administration of multiple IV infusions would benefit both patients and clinicians and should, therefore, be generated. Furthermore, clinicians should be supported with targeted education, establishment of best practices, and bedside clinical decision tools to help them mitigate errors associated with administration of multiple, high-alert IV infusions in the ICU.
Take-Home Points
- The most common types of errors associated with administration of multiple IV infusions in the ICU include: rate or line mix-ups, secondary or Y-site infusion-associated errors, and bolus administration.
- Recommendations for reducing risks of errors associated with co-administration of multiple IV infusions include:
- Utilize a single dosing strategy, either weight or non-weight based
- Setup IV infusions completely and one at a time
- Trace or walk the lines often and when any change in medication administration or line management occurs
- Label lines in a standardized fashion
- Administer high-alert medications as primary infusions
- Utilize infusion sets with back-check valves and multi-port extension sets
- Administer bolus infusions through a primary and isolated/dedicated single access point
- Disconnect and remove all medications/tubing that are no longer ordered
Sarina A. Fazio, PhD, RN
Clinical Nurse Scientist, Center for Nursing Science
UC Davis Health
Emma J. Blackmon, PhD, RN, CCRN
Nurse Educator, Adult Critical Care
UC Davis Health
Amy L. Doroy, PhD, RN, NEA-BC, RN-BC
Nurse Manager, Medical Intensive Care Unit
UC Davis Health
Ai Nhat Vu
PharmD Candidate 2021
UC Davis Health
Paul MacDowell, PharmD, BCPS
Medication Safety Pharmacist
UC Davis Health
References
- Silvestry FE, Manaker S, King TE, Finlay G. Postoperative complications among patients undergoing cardiac surgery. Up-To-Date [database on the Internet] Waltham: UpToDate. 2020.
- Argenziano M, Chen JM, Choudhri AF, Cullinane S, Garfein E, Weinberg AD, Smith Jr CR, Rose EA, Landry DW, Oz MC. Management of vasodilatory shock after cardiac surgery: identification of predisposing factors and use of a novel pressor agent. The Journal of thoracic and cardiovascular surgery. 1998 Dec 1;116(6):973-80.
- Siparsky N, Sterns RH. Overview of postoperative fluid therapy in adults. Obtenido de https://www. uptodate.com/contents/overview-of-postoperative-fluid-therapy-in-adults. 2017.
- Shaefi S, Mittel A, Klick J, Evans A, Ivascu NS, Gutsche J, Augoustides JG. Vasoplegia after cardiovascular procedures—pathophysiology and targeted therapy. Journal of cardiothoracic and vascular anesthesia. 2018 Apr 1;32(2):1013-22.
- Hajjar LA, Vincent JL, Barbosa Gomes Galas FR, Rhodes A, Landoni G, Osawa EA, Melo RR, Sundin MR, Grande SM, Gaiotto FA, Pomerantzeff PM. Vasopressin versus norepinephrine in patients with vasoplegic shock after cardiac surgery: the VANCS randomized controlled trial. Anesthesiology. 2017 Jan;126(1):85-93.
- Osawa EA, Rhodes A, Landoni G, Galas FR, Fukushima JT, Park CH, Almeida JP, Nakamura RE, Strabelli TM, Pileggi B, Leme AC. Effect of perioperative goal-directed hemodynamic resuscitation therapy on outcomes following cardiac surgery: a randomized clinical trial and systematic review. Critical care medicine. 2016 Apr 1;44(4):724-33.
- Manaker S, Parsons P. Use of vasopressors and inotropes. Waltham, MA: UpToDate. 2013.
- Müllner M, Urbanek B, Havel C, Losert H, Gamper G, Herkner H. Vasopressors for shock. Cochrane Database of Systematic Reviews. 2004(3).
- Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Practice parameters for hemodynamic support of sepsis in adult patients in sepsis. Crit Care Med. 1999;27(3):639-60.
- Moran JL, O'Fathartaigh MS, Peisach AR, Chapman MJ, Leppard PH. Epinephrine as an inotropic agent in septic shock: a dose-profile analysis. Critical care medicine. 1993 Jan 1;21(1):70-7.
- Keddissi JI, Youness HA, Jones KR, Kinasewitz GT. Fluid management in acute respiratory distress syndrome: a narrative review. Canadian journal of respiratory therapy: CJRT= Revue canadienne de la therapie respiratoire: RCTR. 2019;55:1.
- Mackenzie DC, Noble VE. Assessing volume status and fluid responsiveness in the emergency department. Clinical and experimental emergency medicine. 2014 Dec;1(2):67.
- Tian DH, Smyth C, Keijzers G, Macdonald SP, Peake S, Udy A, Delaney A. Safety of peripheral administration of vasopressor medications: a systematic review. Emergency Medicine Australasia. 2020 Apr;32(2):220-7.
- Daroca-Pérez R, Carrascosa MF. Digital necrosis: a potential risk of high-dose norepinephrine. Therapeutic advances in drug safety. 2017 Aug;8(8):259-61.
- Van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017 Oct 17;136(16):e232-68.
- Levy B, Clere-Jehl R, Legras A, Morichau-Beauchant T, Leone M, Frederique G, Quenot JP, Kimmoun A, Cariou A, Lassus J, Harjola VP. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. Journal of the American College of Cardiology. 2018 Jul 10;72(2):173-82.
- Pinkney S, Fan M, Chan K, Koczmara C, Colvin C, Sasangohar F, Masino C, Easty A, Trbovich P. Multiple intravenous infusions phase 2b: laboratory study. Ontario health technology assessment series. 2014;14(5):1.
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive care medicine. 2017 Mar;43(3):304-77.
- Cohen MR, Smetzer JL, Tuohy NR, Kilo CM. High-alert medications: safeguarding against errors. Medication Errors. 2nd ed. Washington (DC): American Pharmaceutical Association. 2007:317-411.
- Summa-Sorgini C, Fernandes V, Lubchansky S, Mehta S, Hallett D, Bailie T, Lapinsky SE, Burry L. Errors associated with IV infusions in critical care. The Canadian journal of hospital pharmacy. 2012 Jan;65(1):19.
- Kane-Gill SL, Kirisci L, Verrico MM, Rothschild JM. Analysis of risk factors for adverse drug events in critically ill patients. Critical care medicine. 2012 Mar;40(3):823.
- Institute for Safe Medication Practices (ISMP). Pharmacy–nursing shared vision for safe medication use in hospitals: executive summary session. Am J Health Syst Pharm 2003;60(10):1046-1052
- Fahimi F, Ariapanah P, Faizi M, Shafaghi B, Namdar R, Ardakani MT. Errors in preparation and administration of intravenous medications in the intensive care unit of a teaching hospital: an observational study. Australian critical care. 2008 May 1;21(2):110-6.
- Wollitz A, Grissinger M. Aligning the lines: An analysis of IV line errors. Pennsylvania Patient Safety Advisory. 2014;11(1):1-7.
- Westbrook JI, Rob MI, Woods A, Parry D. Errors in the administration of intravenous medications in hospital and the role of correct procedures and nurse experience. BMJ quality & safety. 2011 Dec 1;20(12):1027-34.
- Schnock KO, Dykes PC, Albert J, Ariosto D, Call R, Cameron C, Carroll DL, Drucker AG, Fang L, Garcia-Palm CA, Husch MM. The frequency of intravenous medication administration errors related to smart infusion pumps: a multihospital observational study. BMJ quality & safety. 2017 Feb 1;26(2):131-40.
- Husch M, Sullivan C, Rooney D, Barnard C, Fotis M, Clarke J, Noskin G. Insights from the sharp end of intravenous medication errors: implications for infusion pump technology. BMJ Quality & Safety. 2005 Apr 1;14(2):80-6.
- Lyons I, Furniss D, Blandford A, Chumbley G, Iacovides I, Wei L, Cox A, Mayer A, Vos J, Galal-Edeen GH, Schnock KO. Errors and discrepancies in the administration of intravenous infusions: a mixed-methods multihospital observational study. BMJ quality & safety. 2018 Nov 1;27(11):892-901.
- The National Coordination Council for Medication Error Reporting and Prevention. New release: Medication Error Council promotes categorization index. 1996 Sept 4.
- ECRI Institute. Top 10 Health Technology Hazards for 2017. November 2016. Available at: https://www.ecri.org/Resources/Whitepapers_and_reports/Haz17.pdf. Accessed March 2021.
- Association for the Advancement of Medical Instrumentation (AAMI). Infusing Patients Safely: Priority Issues from the AAMI/FDA Infusion Device Summit. Available at: https://www.aami.org/docs/default-source/reports/aami_fda_summit_report.pdf Accessed August 2024.
- Institute for Safe Medication Practices (ISMP). ISMP list of high-alert medications in acute care settings. October 2018. Available at: https://www.ismp.org/sites/default/files/attachments/2018-10/highAlert2018new-Oct2018-v1.pdf. Accessed March 2021.
- Calabrese AD, Erstad BL, Brandl K, Barletta JF, Kane SL, Sherman DS. Medication administration errors in adult patients in the ICU. Intensive care medicine. 2001 Oct;27(10):1592-8.
- Ibey AA, Ciarniello C, Gorelik S. Inadvertent overinfusion of NORepinephrine using infusion pump loading dose. Intensive and Critical Care Nursing. 2015 Dec 1;31(6):375-9.
- Pinkney S, Trbovich P, Fan M, Rothwell S, Cafazzo JA, Easty A. Do smart pumps actually reduce medication errors? Human Factors Horizons 2010. 2010;44(s1):64-9.
- Hunter S, Considine J, Manias E. Nurse management of vasoactive medications in intensive care: A systematic review. Journal of clinical nursing. 2020 Feb;29(3-4):381-92.
- Herout PM, Erstad BL. Medication errors involving continuously infused medications in a surgical intensive care unit. Critical care medicine. 2004 Feb 1;32(2):428-32.
- American Society of Health-System Pharmacists. Standardize 4 safety: Adult continuous infusion standards. 2016. https://www.ashp.org/-/media/assets/pharmacy-practice/s4s/docs/Adult-Infusion-Standards.ashx
- Jung B, Couldry R, Wilkinson S, Grauer D. Implementation of standardized dosing units for iv medications. American Journal of Health-System Pharmacy. 2014 Dec 15;71(24):2153-8.
- Tan SY, Said MM, Rahman RA, Taha NA. The effect of education intervention on parenteral medication preparation and administration among nurses in a general intensive care unit. Journal of Pharmacy Practice and Research. 2017 Feb;47(1):8-15.
- Melo EM, Cavalcante HD, Marques AM, Ferreira AM, Abreu MD, Lima VF, Garces TS. Nurses on knowledge vasoactive drugs used in critical patients. J Nurs UFPE online. 2016 Aug;10(8):2948-55.
- Cassano-Piché A, Fan M, Sabovitch S, Masino C, Easty AC, Health Technology Safety Research Team. Multiple intravenous infusions phase 1b: practice and training scan. Ontario health technology assessment series. 2012;12(16):1.
- Furniss D, Back J, Blandford A. Unwritten rules for safety and performance in an oncology day care unit: Testing the resilience markers framework. In Proc. 4th Resilience Engineering Symposium 2011 Jun 8 (pp. 93-99).
- Institute for Safe Medication Practices Canada (ISMP Canada). ISMP Canada Safety Bulletin. What's my line? [Internet]. Toronto: ISMP Canada; 2004 Feb.
- Association for the Advancement of Medical Instrumentation (AAMI). Actions that the healthcare community can do now to improve infusion system safety [Internet]. Horsham (PA): AAMI; Jun 2012.
- Pinkney SJ, Fan M, Koczmara C, Trbovich PL. Untangling Infusion Confusion: A Comparative Evaluation of Interventions in a Simulated Intensive Care Setting. Critical care medicine. 2019 Jul;47(7):e597.
- Institute for Healthcare Improvement (IHI). Best practices for labeling of intravenous lines for patients with multiple simultaneous infusions [Internet]. Cambridge (MA): IHI; 27 April 2011.
- Institute for Safe Medication Practices (ISMP). ISMP Medication Safety Alert. A spectrum of problems with using color [Internet]. Horsham (PA): ISMP; 2003 Nov 13. 4 p.
- Institute for Safe Medication Practices (ISMP). What's my line? [Internet]. Horsham (PA): ISMP; Feb 2004. 3 p.
- Wetterneck TB, Skibinski KA, Roberts TL, Kleppin SM, Schroeder ME, Enloe M, et al. Using failure mode and effects analysis to plan implementation of smart i.v. pump technology. Am J Health Syst Pharm. 2006 Aug 15;63(16):1528-38.
- Institute for Safe Medication Practices (ISMP). Safe Practice Guidelines for Adult IV Push Medications. A compilation of safe practices from the ISMP Adult IV Push Medication Safety Summit. Available at: https://www.ismp.org/sites/default/files/attachments/2017-11/ISMP97-Guidelines-071415-3.%20FINAL.pdf. Accessed March 2021.
- US Food and Drug Administration. Infusion pump improvement initiative. Center for Devices and Radiological Health, Tech. Rep. 2010 Apr.