Blind Spot
The Case
A 36-year-old woman with no significant past medical history underwent right nephrectomy in the left lateral position. The surgery was uncomplicated—her blood pressures intraoperatively were within 20% of her baseline, and she did not have significant blood loss. Immediately after surgery, the patient complained of “blurriness” in one eye. This was attributed to eye ointment applied during the surgery and no further work-up was pursued.
Two weeks later during a clinic visit, the patient complained of blindness in the left eye. She was emergently referred to an ophthalmologist and diagnosed with retinal ischemia as a complication of surgery. Three months after surgery, the patient still had partial visual loss.
The Commentary
This case illustrates the insidious and surprising nature of many cases of postoperative visual loss (POVL). Though not well appreciated by many clinicians, POVL may occur in relatively healthy patients, even when the hemodynamic and surgical course appeared to be uneventful.(1) Although the incidence may be as low as 1 in 61,000 non-ocular surgical procedures, for selected procedures it has been reported to be as high as 1 in 500 patients (after spine procedures) and 1 in 60 patients (after cardiac procedures).(2-4) Because of a perceived increase in POVL, the American Society of Anesthesiologists (ASA) Committee on Professional Liability established the ASA POVL Registry in 1999. This registry collects detailed information on anonymous case submissions of non-ocular POVL and currently contains 129 cases.
Major Postoperative Visual Loss Lesions
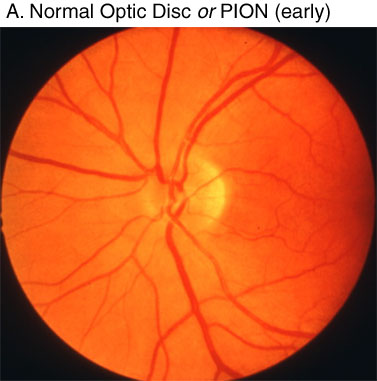
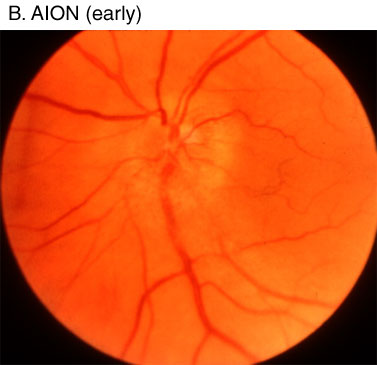
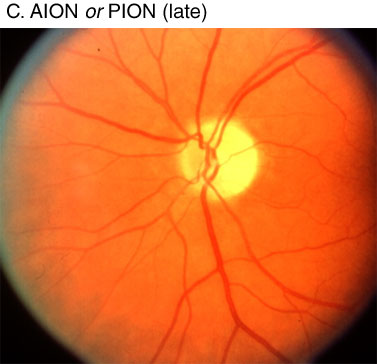
The most common POVL defects are (i) anterior ischemic optic neuropathy (AION), (ii) posterior ischemic optic neuropathy (PION), (iii) central retinal artery occlusion (CRAO), and (iv) cortical blindness. Specific ophthalmologic findings determine the diagnosis.(5) AION occurs at the optic nerve head as the optic nerve and retinal vessels enter the globe. PION occurs anywhere posterior to the optic nerve head to the optic nerve chiasm. Early funduscopic exam will demonstrate disc edema in AION, whereas the fundus is entirely normal in appearance with early PION. Many days to weeks later, when disc edema has usually resolved, the funduscopic exam reveals optic nerve pallor in both AION and PION, and the two entities are indistinguishable at this point (Figure 1). Pupillary light reflexes in AION and PION are either absent or delayed. Patients may complain of total blindness or altitudinal field cuts. In just over half of the ION cases in the ASA POVL Registry, both eyes were affected, consistent with a systemic event.(6) Complete recovery from either AION or PION is rare, and patients are frequently left with a visual field defect, at a minimum.
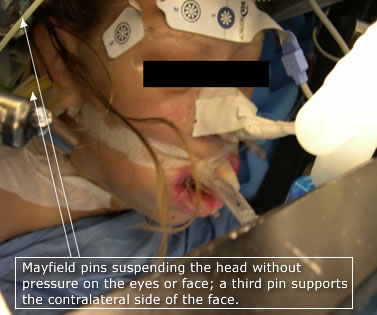
AION has been associated primarily with cardiac bypass procedures but can occur during prone spine operations and other procedures. AION can also arise spontaneously in the community with or without predisposing vascular disease.(7,8) PION is most commonly associated with spine procedures in the prone position and bilateral radical neck operations. Although numerous contributory factors for AION and PION have been proposed (e.g., hypotension, anemia, edema, venous congestion, variations in ocular anatomy and physiology), none has been causally linked in randomized controlled trials or animal models. Data from the ASA POVL Registry have disproved the erroneous theory that ION is caused by pressure on the globe. Of the 43 spine patients with ION from the ASA POVL Registry, eight patients had their heads placed in Mayfield pins (Figure 2) with their eyes free from external pressure.(6) These data clearly demonstrate that ION occurs in the absence of pressure on the globe. Spine surgery cases with ION in the ASA POVL Registry are associated with large operative blood loss (median 2.3 liters) and long duration in the prone position (median 8 hours). Since ION has been reported outside the operative arena in critically ill patients, it seems unlikely that anesthetic agents such as thiopental and volatile agents are significant etiologic factors.(9)
CRAO is the sudden blockage of the blood supply to the retina, often referred to as a “stroke” of the eye. Patients typically present with acute, unilateral, painless visual loss, and they rarely recover any vision. Findings on physical exam consistent with CRAO include an absent pupillary reflex or relative afferent pupillary defect. Funduscopic examination reveals a pathognomonic cherry red spot at the macula, narrowed retinal arterioles, and a pale and edematous retina. CRAO can be caused by globe compression, emboli, venous congestion, and vasculitis. CRAO caused by globe compression is usually associated with periorbital trauma (e.g., bruising, proptosis, paresthesias, or extraocular muscle paresis). The horseshoe headrest (Figure 3) has long been associated with CRAO because of its firmness and close position to the eye with poor accessibility. However, soft foam cushions and gel pads can also cause CRAO if not positioned correctly.
Cortical blindness is the loss of sight due to an organic lesion in the visual cortex. Peripheral vision is frequently affected, but total blindness may occur as well. Both eyes are usually involved, and approximately two thirds of cases may ultimately have some improvement in vision.(5) The physical examination of patients with cortical blindness demonstrates normal pupillary light reflexes with a normal funduscopic exam. Cortical blindness is usually associated with embolic events or profound hypotension and most commonly occurs in association with cardiac bypass operations.
Early Ophthalmologic Exam
The patient in this case was apparently not examined early for visual loss, and we are not supplied with follow-up information, so it is unclear if the patient’s blurry vision in the recovery room was related to her subsequent blindness in one eye 2 weeks later. Worsening or delayed visual loss postoperatively implicates AION as the probable diagnosis. Most cases of PION do not progress in the postoperative period if the patient has stable hemodynamics, and CRAO usually presents with complete loss of vision in the affected eye. Though we lack detailed ophthalmologic exams on this patient, her clinical course most closely resembles AION with progression or onset of visual loss after discharge.
An ophthalmologic consultation and exam with funduscopy should be performed as soon as possible on any patient with POVL to rule out acute angle-closure glaucoma. Numerous drugs given in the perioperative period (e.g., adrenergic agonists, cholinergics, anticholinergics, histamine receptor antagonists) may predispose to this condition. Though rare, it is one of the few forms of POVL that is treatable if therapy is instituted promptly. Moreover, early funduscopic exam allows easier distinction between AION and PION, as the two lesions appear identical at later time points.
Delayed Complaints of Postoperative Visual Loss
Presumably, this patient either had significant worsening, or onset, of her visual loss after discharge. Patients may not alert their clinicians about early POVL, either because of altered mental status or their mistaken beliefs that visual changes are caused by residual anesthetic effects. Occasionally, visual field defects in ION may be subtle, and patients may not recognize or report them until several weeks after discharge. That being said, the occurrence of complete blindness in one eye is sufficiently dramatic and concerning that most patients will report it promptly. Visual loss secondary to AION has been reported to occur anytime from the immediate postoperative period to a few weeks after surgery. Patients with significant gastrointestinal bleeding have also been reported to develop AION between 1 and 3 weeks after their hemorrhage.(10) Alternatively, an unremarkable hospital course, as in this case, leaves open the possibility that the development of AION in some patients may be coincidental timing and not necessarily related to “perioperative stress.”
The finding of retinal ischemia in this patient 2 weeks postoperatively is most likely a misinterpretation of the findings of optic nerve ischemia. Perioperative ION is best diagnosed by either neuro-ophthalmologists or ophthalmologists who specialize in ION. The actual funduscopic findings, which we are not provided, are essential to making a diagnosis of the lesion. Although retinal ischemia is a finding consistent with CRAO (and not with AION), CRAO is not associated with progressive loss of vision. However, we cannot entirely rule out the possibility that the patient had complete blindness in one eye that went unreported for 2 weeks, making CRAO a remote possibility in this case.
Diagnostic Studies for POVL
Screening for POVL after surgery is usually reserved for high-risk procedures such as head and neck procedures, cardiac bypass, and major spine operations, and generally involves gross testing of vision in each eye. Complaints of even mild visual changes after any procedure should be followed up with ophthalmologic consultation if symptoms persist once the patient is alert and able to provide a reliable exam. Ophthalmologic exam is the only essential test for the workup of POVL and should include testing for visual acuity, intraocular pressures, color discrimination, gross visual fields, pupillary reflexes, and funduscopy with pupillary dilation. The ability to read letters and numbers or count fingers does not preclude the possibility of scotoma or discrete visual field deficits as may occur in ION. If possible, formal visual fields performed in the ophthalmologist’s office prior to discharge may be helpful for determining the extent of the visual defect and provide a baseline comparison for future exams.
MRI may demonstrate occipital cortical strokes in the event of cortical blindness, but it is not currently useful for the diagnosis of ION. Electroretinograms are sensitive detectors of retinal ischemia and will detect CRAO, but these are rarely used, as funduscopic exam is sufficient for this diagnosis. Visual evoked potentials (VEPs) detect abnormalities of the optic nerve and its projections to the occipital cortex. VEPs are useful in the diagnosis of ION before optic nerve pallor has emerged if visual acuity cannot be assessed. Currently, VEPs are unreliable when used in the presence of general anesthesia, whether volatile or total intravenous-based, and therefore have not been utilized as an intraoperative monitor of optic nerve function.(11)
Treatment
In the case presented here, an earlier exam and follow-up might have been helpful in diagnosis, but it is unlikely any meaningful treatment would have been possible. High-dose steroids, hyperbaric oxygen treatment, mannitol, and furosemide have been utilized for treatment of ION with inconsistent results. Restoration of the mean arterial pressure to baseline and treatment of significant anemia are frequently suggested interventions by consultants, regardless of the diagnosis. However, no randomized trials are available to document the efficacy of any intervention.
Prevention
What can be done to prevent POVL? Data delineating specific risk factors are lacking, making the implementation of preventive measures difficult. However, certain steps seem sensible. CRAO caused by pressure on the globe can be prevented by careful and frequent attention (with documentation) to the patient’s eyes when positioned prone or when objects are close to the eyes. Mayfield pins are an alternative to the horseshoe headrest in the prone position when the eyes cannot be adequately protected or frequently checked during the procedure. The usual safeguards to prevent air emboli (e.g., make operative site lower than heart when possible to reduce venous pressure gradient; de-air intravenous fluid bags and have air filters for rapid infusion devices; consider an air-aspiration central venous catheter for high-risk procedures) should be employed to prevent cortical blindness. However, many of these cases are thought to be caused by particulate emboli and thus may not be easily preventable. Because the etiology of ION is unproven, any interventions should be evaluated in terms of both their potential efficacy and their potential to cause harm.
Take-Home Points
- Postoperative visual loss is an uncommon, but not rare, complication of surgery, particularly for spine and cardiac surgery.
- Patients with postoperative visual loss should be evaluated by an ophthalmologist as quickly as possible.
- Isolated ION is not caused by pressure on the globe.
- ION has many proposed risk factors, but none have been proven; thus, there are no evidence-based prevention strategies.
- Postoperative visual loss caused by pressure on the globe results in CRAO and is usually associated with other signs of periorbital trauma. Frequent eye checks with documentation should be performed intraoperatively for all procedures performed with the patient in the prone position.
- Mayfield pins will prevent CRAO from pressure on the globe, but not ION. The horseshoe headrest makes it difficult to perform adequate eye checks and provides little margin for head movement without compressing the ocular globes.
- Except for acute angle-closure glaucoma, there are no proven beneficial treatments for postoperative visual loss.
Lorri A. Lee, MD Director, ASA Postoperative Visual Loss Registry Assistant Professor, Department of Anesthesiology University of Washington
References
1. Lee LA, Lam AM. Unilateral blindness after prone lumbar spine surgery. Anesthesiology. 2001;95:793-795. [ go to PubMed ]
2. Roth S, Thisted RA, Erickson JP, Black S, Schreider BD. Eye injuries after nonocular surgery. A study of 60,965 anesthetics from 1988 to 1992. Anesthesiology. 1996;85:1020-1027. [ go to PubMed ]
3. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine. 1997;22:1319-1324. [ go to PubMed ]
4. Breuer AC, Furlan AJ, Hanson MR, et al. Central nervous system complications of coronary artery bypass graft surgery: prospective analysis of 421 patients. Stroke. 1983;14:682-687. [ go to PubMed ]
5. Roth S, Gillesberg I. Injuries to the visual system and other sense organs. In: Benumof J, Saidman L, eds. Anesthesia and perioperative complications. 2nd ed. St. Louis, MO: Mosby; 1999:377-408.
6. Lee LA. ASA postoperative visual loss registry: preliminary analysis of factors associated with spine operations. ASA Newsletter. 2003;67:7-8. Available at: https://www.aqihq.org/ClosedClaimsPDF/Click%20here%20for%20_62.pdf Accessed August 29, 2024.
7. Hayreh SS, Joos KM, Podhajsky PA, Long CR. Systemic diseases associated with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1994;118:766-780. [ go to PubMed ]
8. Characteristics of patients with nonarteritic anterior ischemic optic neuropathy eligible for the Ischemic Optic Neuropathy Decompression Trial. Arch Ophthalmol. 1996;114:1366-1374. [ go to PubMed ]
9. Lee LA, Nathens AB, Sires BS, McMurray MK, Lam AM. Blindness in the intensive care unit: possible role for vasopressors? Anesth Analg. 2005;100:192-195. [ go to PubMed ]
10. Hayreh SS. Anterior ischemic optic neuropathy. VIII. Clinical features and pathogenesis of post-hemorrhagic amaurosis. Ophthalmology. 1987;94:1488-1502. [ go to PubMed ]
11. Wiedemayer H, Fauser B, Armbruster W, Gasser T, Stolke D. Visual evoked potentials for intraoperative neurophysiologic monitoring using total intravenous anesthesia. J Neurosurg Anesthesiol. 2003;15:19-24. [ go to PubMed ]
Figures
Figure 1. Funduscopic exam of (A) normal fundus and early posterior ischemic optic neuropathy (PION)—early PION has completely normal exam; (B) early anterior ischemic optic neuropathy (AION) demonstrating narrowed retinal arterioles and blurred optic disc margin/swelling; and (C) late AION and PION demonstrating optic nerve pallor and loss of disc edema. Source: Hayreh SS. Clinical features of non-arteritic and arteritic AION. Available at Accessed June 6, 2005. Reprinted with permission from Dr. Hayreh.
Figure 2. Patient undergoing major spine surgery in the prone position with the head in Mayfield pins and the eyes free from pressure. Note facial edema, which is present to some degree in all major procedures in the prone position.
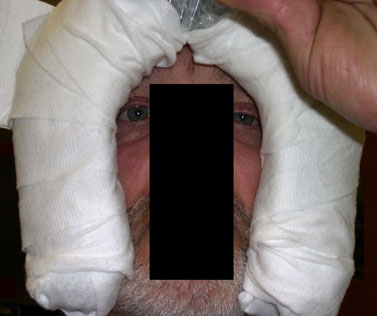
Figure 3. The horseshoe headrest’s design puts the majority of the pressure of the face on the outer rim without utilizing the chin or midface (i.e., cheeks/maxilla) for pressure support. There is a very small margin between the headrest and the eye. If the face is moved on the headrest during surgery, significant pressure can be applied to one eye.